Egg-cellent Easter Science
The Naked Scientists take a special holiday look at some egg-cellent Easter science, including a breakthrough in how to unboil an egg, the genetically modified chickens that can't catch bird flu and why the Easter bunny might be knocked off his perch by a toucan. Plus, is a chocolate teapot really useless?
In this episode

00:50 - How to unboil an egg
How to unboil an egg
with Professor Greg Weiss, University of California, Irvine
Eggs are a familiar symbol of Easter, both chocolate and boiled. Many people celebrate Easter Sunday with a boiled egg with a face on it. The familiar change that takes place when we boil an egg - the runny egg white goes rubbery and becomes opaque- occurs because heat causes the proteins that make up the egg white to change their shape - or denature. Now, University of California, Irvine, scientist Greg Weiss has found a way to reverse the process or "unboil" an egg. And this could be a very useful technique for mass-producing proteins that we need to study, or for medical use, as he explained to Chris Smith...
Kat - To start off, one of our producer Georgia's favourite Easter traditions is to draw happy faces on her soft-boiled eggs before ruthlessly decapitating them and dipping toast in their brains.
Chris - Yumyum! Now, the familiar change that takes place when we boil an egg - that runny white going rubbery and becoming white - it occurs because heat causes the proteins that make up the egg white to change their shape or do what we call denature. Now, University of California Irvine scientist Greg Weiss has found a way to reverse this process, or as he puts it, "unboil" an egg. This could be very useful for mass-producing proteins that we need to study, or for medical uses.
Greg - We didn't originally set out to unboil eggs. Originally, I just wanted some way of untangling proteins that get mashed together and folded up and agregated. So, I was in the lab one day and we had this new method of yanking on the protein chains and forcing them into their correct shapes. I realised that no one on the planet would believe how cool this was until I could demonstrate this on a problem that everyone acknowledges as hard. And then suddenly, I realised, that egg that I had for breakfast would be perfect.
Chris - First of all, tell us what is a protein and why is this important?
Greg - Proteins are the workhorses of your cells. They're the things that make you do the things you do, that raise your arms, that provide the muscles, that run around the body and do all kinds of things. Those proteins are absolutely fascinating little machines that scientists like myself want to study. So, when we start looking at them in detail and start examining their functions and how they work, oftentimes, we have to produce them in big quantities because only small quantities are available inside the cells. So, we produce a big batch of these things and start examining them in detail. But the problem is, when you're making these big batch of stuff, it's kind of like making a big batch of chocolate chip cookies or something. It doesn't come out the way the small batch did. They come out messed up, tangled together, just like egg whites after you boil them.
Chris - So, when I do boil my egg, the runny stuff, the white becomes white, doesn't it? So, what's actually happened to make a cooked egg go white like that?
Greg - The boiled egg, proteins inside the egg white are slipping apart. And so, instead of being like neatly folded up sheets, let's say, just sheets that you put on your bed, instead of being nicely folded, they get thrown around the room inside the eggshell. You get a big pile of them that are tangled together and that tangled mass appear white to us.
Chris - But the bottom line is that the proteins that were in the egg white when it was in the runny, uncooked state, and now, in a very different shape and state once we have boiled the egg.
Greg - Eggs-actly!
Chris - The pun didn't escape me there, Greg.
Greg - Sorry.
Chris - So, what has happened?
Greg - So, the proteins get tangled together and then that's what appears white to us. In that state though of tangled up, those proteins are useless. You know that because even if we fertilised the boiled egg, nothing would happen, right? When proteins are tangled up, they're not in their natural shapes and they can't do their natural functions. We needed a way of getting them out of that tangled up shape so that we can look at them in greater detail in laboratories like my own which focuses for example on cancer research.
Chris - So, how did you get the egg to unboil itself then?
Greg - We started with eggs that were purchased from the local grocery store, brought them home and then separated out the yolks from the whites - using of course, Julia Child's classic methods - boiled the egg for a good 10 to 20 minutes so a really long time. So, these were hardboiled eggs, like hard, hardboiled eggs. And then we took the hardboiled egg, dissolved it in a chemical called urea. So, urea is this fascinating chemical that gets into the protein tangles and starts breaking apart the little tiny contacts they have with each other. At that point, the proteins are still totally useless. They're like a tangled mass of sheets that have been thrown on the ground. We then put them in a special device called the vortex fluid device. The proteins are subject to tremendously high shear stress as they're whirled in a tiny mini centrifuge. In this shear stress, the proteins are pulled and unpulled like rubber bands. And then spontaneously, they start folding up into their correct shapes.
Chris - So, when you put them into the laboratory equivalent of a food mixer - that centrifuge essentially - you're putting them under a shear stress or you're pulling on the proteins and that makes them sort of untangle, unthread themselves from this bundle that you had before. And then they're given the opportunity to put themselves back to the shape they had before we boiled the eggs.
Greg - That's correct.
Chris - How do you know that the proteins do go back to their egg unboiled state?
Greg - Yes, good question. So, there are a very large number of proteins inside an egg white, but we examined the abilities of one protein that's a champion at chewing on any bacteria that happen to get pass the eggshell. Normally, after you boil eggs, this protein is totally messed up. But after our unboiling experiment, the protein returns to 80% or so of it normal activity. To demonstrate that, we basically fed it these bacteria cells and then watched it chewing on their cell walls.
Chris - Goodness! So that suggests that it would actually work for a whole range of different proteins in the egg but then also, potentially other tangled up denatured proteins in other circumstances in the laboratory for example.
Greg - Yes. That's precisely what we want to do, is to apply this now to proteins that are associated with cancer, proteins that we'd like to use as flags for cancer to develop better diagnostics, proteins that we want to study their structures and understand how mutations to these proteins drive cancer and drive tumour formation.
Chris - Does it work on chocolate eggs?
Greg - You're the very first person to ask. I'm tempted to go back to the lab right now, but that's only because I just want to purchase a large amount of Cadbury eggs and claim that it's a lab expense.
Chris - Sounds like a very fun experiment. Greg Weiss, unboiling eggs including possibly, chocolate ones. And on the subject of experimenting, over to Kat...

07:17 - Eggsperiment 1: Spinning eggs
Eggsperiment 1: Spinning eggs
with Georgia Mills, The Naked Scientists
Ever been dared to smash an egg over your head? Georgia Mills showed Kat  Arney a handy trick to find out if the egg is raw or boiled, without getting egg on your face...
Arney a handy trick to find out if the egg is raw or boiled, without getting egg on your face...
Georgia - This is a way to tell if your egg has been cooked without having to smash it open and potentially get raw egg everywhere.
Kat - So, what have we got here? We've got two eggs on the table. They both look exactly the same. Is one of them cooked and one of them not cooked?
Georgia - One of them has been hardboiled for 6 minutes and the other one is complete fresh from the chicken.
Kat - How do we tell the difference between the two?
Georgia - What I'm going to do is I'm going to spin both eggs around on their sides on the table really quickly. I'm going to put my finger on to stop them and then take it off again straightaway.
Kat - Wow! One of them has stopped dead but one of them readily has started rotating again. So, what's the difference between them?
Georgia - Well, the one that kept moving, that's the raw egg. What happened there is that the liquid inside kept its momentum even when I stopped the shell. When I let go again, the momentum caused friction on the inside of the egg and started it moving again.
Kat - That is pretty cool. I mean, if I ever have an egg-based dilemma in my fridge. But is there anything else that we could use this kind of knowledge for about momentum of fluids?
Georgia - Well, it's interesting because this is exactly what causes you to feel dizzy when you spin around because the liquid in your ear keeps its momentum even after you stop. It's really good to use this for when you're thinking about transporting liquid as well. If you have one big container, the liquid inside will keep its momentum and slush up and down and cause things like Lorries to flip up on their side. But if you have small compartments throughout the lorry, this will not happen so much.
Kat - So, I don't have a handy oil tanker but we do have some eggs and I think I will pick the hardboiled one for my tea.

09:10 - The Easter bunny
The Easter bunny
with Dr Jon Bielby, Zoological Society of London
Easter Eggs are traditionally distributed by the benevolent Easter bunny. But  there's one problem, where would a rabbit find an egg? They certainly don't lay them, but why not? Graihagh Jackson took a trip to London Zoo to search for an answer...
there's one problem, where would a rabbit find an egg? They certainly don't lay them, but why not? Graihagh Jackson took a trip to London Zoo to search for an answer...
Graihagh - I have four beautiful rabbits - Walnut and Hazel, Smudge and Pushkin. I've been wondering for a long time whether they might surprise me one year and lay a chocolate egg for Easter because that's what the Easter bunny does, right? Well, maybe not and at least it's not only my pet rabbits that aren't grateful enough to lay me an egg. Of course, there's a huge evolutionary diversity in the animal kingdom when it comes to reproduction. In team one, you have the live-birthers and in team two, the egg layers. One of the top players in team 2 are the birds. But why do some animals lay eggs and others give birth? What's better, and who does what? To get the egg rolling, I played a game with ZSL zoologist Jon Bielby in the birdhouse of London Zoo. The game is, birther or egg layer. Winner gets a gold star. Ready, set, go...flamingo.
Jon - Egg.
Graihagh - Giraffe.
Jon - That will be a live young.
Graihagh - Panda.
Jon - Live young.
Graihagh - Penguin?
Jon - Eggs.
Graihagh - Duckbill platypus.
Jon - That's a tricky one. It's a mammal, but it lays eggs.
Graihagh - So, there's sort of a 50/50 split there between live young and eggs. Is that the same across the entire animal kingdom or is it less evenly distributed than that?
Jon - Across the whole animal kingdom, it would be less evenly distributed than that. You'd have many, many more animals that lay eggs rather than giving birth to live young.
Graihagh - These two main varieties, what's the benefit? Why does some animals lay eggs and others give birth to live young?
Jon - The main reason is that there are lots and lots of different ways to make a living. So, we're walking around London Zoo, you see tons of different ways that animals do things - sizes, diets and that includes how they breed, how they reproduce and what their young do. We think of all of vertebrates, all the things with backbones so fish, amphibians, reptiles, birds, mammals. The default setting is laying eggs and that's because the ancestor of all of those different types of animals laid eggs. But at various points across that family tree giving to live young has evolved in different ways. So, both are equally successful. Both allow animals to pass on their genes and that's what natural selection and evolution is all about really.
Graihagh - If we all started as egg layers - I'm laughing at the image of myself laying an egg - why would we suddenly evolve and across so many different animal kingdoms as well, how did that happen if you like?
Jon - The reason why it might happen is that a population or individuals within a population would have a mutation to their genetics. What it meant is that instead of laying eggs, they'd maintain their eggs for a little bit longer in their oviduct and then they'd lay eggs that would hatch ouy more quickly and so on and so forth. And if they survive better then their genes would be passed on to the next generation.
Graihagh - Is there any examples of it going the other way around say, from live birth back to laying eggs?
Jon - As far as I'm aware, it's not gone the other way as yet.
Graihagh - But what about the Easter bunny because he or she famously carries around chocolate eggs? Jon and I sauntered down to the rabbit enclosure to find out if this myth could possibly be true. Could my pet rabbits lay me an egg this Easter?
Jon - I don't think you're going to go home and find that your rabbits have laid eggs. Have rabbits ever laid eggs? Sorry, no, I don't think so.
Graihagh - Would they ever evolve to lay eggs in the future?
Jon - I think it's really, really unlikely. There are a couple of reasons for that. One is that if you think of how different laying an egg is, compared to giving birth to live young. So, if you look at your egg that you might have for breakfast, you crack it up and you've got the yellow bit which is the yolk and then there's the white bit which is a protein. The shell itself is really specialised piece of equipment if you like for looking after a chick. In a rabbit, what would happen is that the young rabbits inside the mother would feed through the placenta. Think of a placenta compared to an egg. It's really, really different. Now, the first reason I think is, it's really unlikely that you're going to go home and find that your rabbits have laid eggs is that having evolved all of this really elaborate way of looking after the young via a placenta, it's really unlikely that the body is physiologically going to be able to change and go back to lay an egg. I don't know whether that's impossible or not but if you think about if that happened over thousands or even millions of years, it wouldn't really be a rabbit anymore. It would be something else. But the other reason is that rabbits are really successful, so that really need to do that. Rabbits breed like rabbits. There are millions of them. So, there's not really the pressure for them to go back to laying eggs.
Graihagh - You've just smashed on my hopes and dreams of the Easter bunny ever coming to visit.
Jon - I'm really sorry about that. I mean, you might still get some Easter eggs this week and I'm not sure. But yeah, probably not laid by a rabbit.
Graihagh - So, did the Easter bunny steal his eggs from the chickens then? Is that what happened?
Jon - It's possible, but as far as I know, the eggs that the Easter bunny delivers are chocolate. As far as I know, chickens don't lay chocolate eggs. I'm not sure how that happened biological speaking.
Graihagh - There aren't animals that lay chocolate eggs I'm assuming.
Jon - It would clearly melt inside of the oviduct, so it's just not going to happen. I suppose maybe that's why they got protective tinfoil insulation or something.

14:43 - Eggsperiment 2: Naked eggs
Eggsperiment 2: Naked eggs
with Georgia Mills, The Naked Scientists
Eggs shells are notoriously tough and brittle, but there is a way to remove the  hard parts while leaving the egg intact. All you need is a fair amount of vinegar and 24 hours, as Georgia Mills explained to Kat Arney...
hard parts while leaving the egg intact. All you need is a fair amount of vinegar and 24 hours, as Georgia Mills explained to Kat Arney...
Georgia - Well Kat, you're a Naked Scientist, so I thought I'd make you a naked egg. If you want to do this at home, all you need is a normal egg and a cup of vinegar and you need to put the egg in the vinegar for about 24 hours.
Kat - Do you need to have it completely covered by the vinegar?
Georgia - Completely submerged, yes. I made one earlier and I've got it in this small container here. so, if you'd like to open it up and look inside...
Kat - Okay, so right straightaway, I've got the really pungent smell of vinegar in my nostrils. There's all this kind of weird foam, kind of scummy foam on the top and I can see bubbles all over the egg, all over the egg shell. I'm going in, I'm going to pick it up. AHHH, vinegar everywhere! Right, it's egg-shaped. It's rather anaemic egg colour, but it doesn't feel like an egg. This is kind of squishy and soft. It feels almost like one of those kind of gummy toys that you use to get. What's going on here?
Georgia - Like I said, this is a naked egg. So, it's lost the hard part of its shell. This is because vinegar is an acid and the shell is made of calcium carbonate. When this two go together, they make a reaction which creates carbon dioxide. That's the bubbles you saw.
Kat - So, the carbon dioxide explains the bubbles but what about that weird soft shell? What's happened there?
Georgia - Well, a shell is made of this calcium carbonate scaffolding with the egg membrane inside it. What we've done is we've taken away that scaffolding because it's reacted with the vinegar to form calcium acetate which is dissolved in the rest of the liquid, leaving behind a squishy, gooey membrane.
Kat - I guess if chickens are making these hard calcium carbonate eggs, they must need to have a lot of calcium or something. Do they eat chalk or something?
Georgia - As far as I know, they don't eat chalk but they do have to eat a lot of extra calcium. In fact, when birds themselves are ovulating, they tend to borrow calcium from their bones. You can actually tell what gender a bird is or even a dinosaur because they are ancient birds really, just from looking at their bones.
Kat - When I went to the Natural History Museum to meet Sophie the Stegosaurus a couple of weeks ago, yeah, the person who was working on her bone said, "Oh, we can tell that she wasn't ovulating because she didn't have those characteristic signs." So fascinating stuff but meanwhile, I've got one hardboiled egg for my tea and do you know what Georgia, I don't think I'll have that one, thanks.
Georgia - That's one for me then.

17:19 - Flu-resistant GM chickens
Flu-resistant GM chickens
with Dr Laurence Tiley, University of Cambridge
Where would Easter be without chickens? There are something like 30 billion of  them on Earth, and with these sorts of numbers, bird flu is a big concern. Not only can it wipe out a farmer's livelihood, it can also spread to humans and cause a pandemic. Biologist Laurence Tiley thinks he has the ideal Easter treat - a genetically modified hen that can't contract the flu bug. At the moment they're an alarming green colour, but he's working on that...
them on Earth, and with these sorts of numbers, bird flu is a big concern. Not only can it wipe out a farmer's livelihood, it can also spread to humans and cause a pandemic. Biologist Laurence Tiley thinks he has the ideal Easter treat - a genetically modified hen that can't contract the flu bug. At the moment they're an alarming green colour, but he's working on that...
Laurence - Flu is a virus, which means it's a very tiny organism that's completely dependent on infecting a cell and replicating within the cell of a host. It's not like a bacteria, which it can sort of free-live. It has to actually get into an animal in order to reproduce itself. Flu causes disease in a lot of different species - horses, pigs, chickens are the ones that most people know about.
Chris - How much of a headache is flu for farmers of chickens?
Laurence - It can be a big headache. Strains that are, what we call highly pathogenic influenza viruses, they will kill 99% of a farmer's birds in a space of a couple of days. So, we're trying to make strains of chickens that are intrinsically resistant to influenza or infection.
Chris - They can't catch flu.
Laurence - That's the ultimate goal, yes. We've made progress towards that. We've got birds that although they can still be infected, they don't pass the infection on to other birds.
Chris - How?
Laurence - By putting a new gene into the bird that produces something that throws a spanner in the mechanics of the virus. It cums up the ability of the virus to copy itself and transmit on from one cell to another. The virus has to copy itself in order to reproduce and be able to cause an infection and spread from one cell or one animal to another. The way it does that, it uses an influenza virus protein and enzyme that can copy and make new versions of its genetic material that it can then package up into new particles and those new viruses. So, we've introduced something into the genome of the chicken that produces a small molecule that looks like a flu genome. When the virus infects those cells, it gets diverted into copying this little decoy rather than copying its own genome.
Chris - So, the virus infects the cell. Because you've got your little thing that looks a bit like a virus component sitting in the genetic material of the cell, this also starts being copied. It side-tracks the cell into focusing on that rather than on making viable flu viruses in that cell.
Laurence - That's the theory behind, yes.
Chris - Have you actually done this yet? Have you got chickens that are making this molecule?
Laurence - Yes, we've made chickens that have got a new gene inserted into them and that produces this particular molecule. We've tested them and shown that it does interfere effectively with the transmission of the virus from one bird to another.
Chris - Will this work for all of the different strains of flu because one of the things about flu is it comes in many different flavours? You hear about H1N1 and H3N2 in humans. Birds have maybe 17 of these different combinations, don't they? So, will your thing work against any type of flu that comes along?
Laurence - I think that's the beauty of this particular approach that we're taking is that the little bit of genetic material that we're introducing looks like this piece of flu, is absolutely the same in all of those strains of flu. It's a very, very important control element for the virus so there's very limited opportunity for it to evolve around it.
Chris - What about safety? Because people are very worried particularly in the wake of things like BSC, which is another example of things getting into the food chain that people would rather not had in the food chain. Is there any risk to the people who might eat these chickens?
Laurence - No. the nature of the molecule that we've introduced into the chickens, it's not a protein for example. So, there would be no chance of anybody developing an allergy to it. The type of molecule it is would not be recognised by our immune system. It would have absolutely no effect on somebody who ate it and the chickens are producing this in every single cell of their body and they're absolutely normal, except that they're bright green because we use a fluorescent marker to track which ones are genetically modified and which ones aren't. Clearly, when it comes to the human food chain, we wouldn't be trying to flog fluorescent green chickens.
Chris - I don't know, a glowing green chicken might make quite a good marketing gimmick. That was Laurence Tiley from the University of Cambridge.

21:37 - As useless as a chocolate teapot: part 1
As useless as a chocolate teapot: part 1
with Dave Ansell and Ben Valsler, Naked Scientists
How often have you heard the saying "as useless as a chocolate teapot?"  Probably quite often. But is it really useless? We dispatched Naked Scientists Ben Valsler and Dave Ansell to find out...
Probably quite often. But is it really useless? We dispatched Naked Scientists Ben Valsler and Dave Ansell to find out...
Ben - Why is it that a chocolate teapot would be so useless?
Dave - Well the reason why chocolate is so nice to eat is that it's the point at which it melts is very similar to the temperature of your mouth. So, it should melt in your mouth. So, as you're going to put boiling water into it, it should melt really quickly and just collapse.
Ben - So, I guess what we need to find out is how thick the walls of the chocolate teapot need to be in order to not melt through by the time the tea is brewed. How are we going to find this out?
Dave - We could just make lots of chocolate teapots but that will take ages and use a really ridiculous amount of chocolate. So instead, what I've got is a whole series of tubes with different amounts of chocolate in the bottom of each one and then pour boiling water over them and see how long they take to melt.
Ben - So, the tubes aren't actually sealed at the bottom. There's just a plug of chocolate in there of different thicknesses. In theory, at least one of these thicknesses of chocolate should stay solid long enough to brew a cup of tea.
Dave - Well, because I've got no idea how thick this has got to be, I've gone from very wide range of different thickness of chocolate from about 10 mm up to about 80 mm. So, one of those ought to survive the brewing time.
Ben - It looks to me like you've chosen to go for dark chocolate.
Dave - Milk chocolate's got a lot of milk fat in it and milk fats got lower melting temperature than coco fat. So, dark chocolate ought to melt at high temperatures. So, it would be slightly better than milk chocolate for these purposes.
Ben - Okay, so I guess the first thing we need to do in that case is boil a kettle and pour some water into each of these tubes.
Dave - I'll go and get that kettle.
Ben - So, while Dave goes to get the kettle, I'm just going to have a look at these tubes of chocolate and they are just plastic tubes open at both ends with a thick plug of chocolate. I don't think these are going to last very long when we pour boiling water in. here comes Dave now with a steaming hot kettle so we can try it out. So Dave, are you going to put them in any particular order?
Dave - Well, I figured I'd start on the thickest plug of chocolate first because that should last the longest. So, it's probably the fairest to start with that one. I'll try and put that same amount of water on top of each one roughly so it's a fair test.
Ben - So, there isn't a different weight of water that could just push the plug of chocolate out.
Dave - Yeah, that's the idea.
Ben - So, what else is in chocolate? I know you've already mentioned coco fats and milk fats but surely, there's more to it than that.
Dave - Well, a good quality dark chocolate is basically just coco fat, some coco solid which are the things which aren't fat from a coco bean and lots of sugar.
Ben - Okay, well there's just been a bit of activity with these tubes. The tube with the thinnest plug of chocolate in has just dropped some chocolate onto the floor and emptied itself. In fact, that's really not a very pleasant thing to watch the way it's just oozing chocolate into a dollop on the floor. Dave, how long did that take?
Dave - That seems to have been about 4 minutes which is not bad for only a 10-mm piece of chocolate. It's a lot better than you'd expect really.
Ben - And 4 minutes is actually about as long as you need to brew a cup of tea.
Dave - Indeed. So, maybe chocolate is a better teapot material than we'd first thought.
Ben - So now that we know that a thickness of chocolate which is only about 10 mil or so, about a centimetre, should be thick enough to make a teapot out of. Shall we make a teapot?
Dave - Yeah, I think so. We'll probably make one with rather thicker walls than that because a teapot is quite a lot bigger than the 40-mm tubes we're using. So maybe we'll go for about 20-mm wall and see what happens.
Ben - Excellent! And I do like my tea quite strong so it will be good to be able to brew it for that a little bit longer. So, Dave and I are now going to go into his kitchen and make ourselves a chocolate teapot. We'll come back to you later on the show to let you know how good it was at brewing some tea.

25:16 - The plant that resurrects
The plant that resurrects
with Dr Bruce Roser, StablePharma
A key symbol of the Easter holidays is resurrection. Christians may celebrate a  famous resurrection on Easter Sunday, but according to the Bible, Jesus definitely wasn't brought back to life by dunking him in water. However, that's exactly how a new breed of preserved vaccines and drugs are resurrected, after being held in suspended animation in a special sugary glass - a discovery that could revolutionise vaccine delivery across the world. Kat Arney spoke to Cambridge-based scientist Bruce Roser to discover how the technology works.
famous resurrection on Easter Sunday, but according to the Bible, Jesus definitely wasn't brought back to life by dunking him in water. However, that's exactly how a new breed of preserved vaccines and drugs are resurrected, after being held in suspended animation in a special sugary glass - a discovery that could revolutionise vaccine delivery across the world. Kat Arney spoke to Cambridge-based scientist Bruce Roser to discover how the technology works.
Bruce - All vaccines and many drugs are stored in the refrigerator. Absolutely, it have to be stored in the refrigerator because outside of the refrigerator, they're unstable. This is particularly true of vaccines and particularly a problem of vaccines because they have to be shipped all over the world to remote places without access to refrigeration. It’s an enormous job for the WHO to put a mine of refrigerators all the way from the factories in the west to remote towns in Africa. So, that’s the problem. How do you stabilise vaccines and other drugs so this is not required?
Kat - Where did you look to for your inspiration?
Bruce - The inspiration came out of the blue. I was asked to make a presentation at the Royal Society and one of my problems was that the reagents I was using are very unstable and a colleague suggested to me that I read a paper about how to address unstable proteins with simple, natural process. So, I read this up and I discovered these living organisms called anhydrobiotic organisms that were able to dry out during a drought and then come back to life again when water was added back to them even after a hundred years. These are things like the resurrection plant. There are also some animals that do this and of course for example, baker’s yeast. You can buy those dried powder in the supermarket, add water to it and it comes back to life.
Kat - Very appropriate if anyone’s been making hot cross buns this weekend I suppose.
Bruce - Exactly, yes.
Kat - Tell me a little bit more about what's going on in these organisms to enable them to preserve themselves for so long.
Bruce - When I started to study this issue, two things were noted by previous people where one had – these organisms could dry out and come back to life and the second is that they contained a lot of the very unusual sugar called trehalose. Being completely in this entire thought, well obviously, trehalose causes this phenomenon whereas almost anybody else who knew anything about it said this is much too complicated process for it just to be due to a simple sugar. But I did some experiments and found that the simple sugar would exactly replicate what was happening in these organisms. It turns out that the process is very beautiful and very simple. When you dry these organisms, all the sugar that they have inside their tissues doesn’t crystallise as the animals dry out the way normal sugar crystallises. But it becomes more and more viscous like honey which would’ve turned into a thick syrup. As you remove more and more water, it gradually solidifies into what's called a glass. These glasses are made of sugar so that they dissolve very easily in water. the molecules that were floating about in these organisms while they were alive become trapped in this viscous sugar solution and eventually in the glass so that they can't move about, they can't interact with each other and therefore, they can't deteriorate. So, they're trapped in suspended animation if you like in the sugar glass.
Kat - It’s a wonderful image of all these little molecules just trapped forever, almost like Han Solo frozen in time in his carbonite.
Bruce - Exactly. A better principle is probably some of the living insects that you see trapped in amber. These all happens at room temperature or above. You don’t have to use any freezing at all. Of course, that’s why it’s so valuable for vaccines in the developing world.
Kat - If all you need to do this process is some molecules, some living things and some sugar, does it work for everything? Could you make it work for any vaccine you wanted?
Bruce - Yes. We’ve done about – I think it’s about 20 vaccines and it works with all of them. We don’t have much doubt that it will work with anything that’s given to us.
Kat - We’ve heard so much in media over the past year about the Ebola outbreak in western Africa. Do you think that potentially, Ebola vaccines could be preserved in this kind of way too?
Bruce - What I know about Ebola vaccines, they are the sort of vaccines that we’ve worked with before, with which the technique works perfectly.
Kat - It does sound absolutely fantastic. So of course, the big question is, how soon? When can you start getting this kind of preservation technique out into the world?
Bruce - We’ve got to the stage now where we’re in animal trials. So, we’re hopeful that if our animal experiments go well, we should be able to get approved in humans very quickly. So, we’re talking about 2 to 5 years, hopefully, closer to 2.
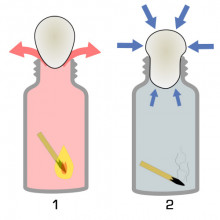
30:45 - Eggsperiment 3: An egg in a bottle
Eggsperiment 3: An egg in a bottle
with Georgia Mills, Naked Scientists
This time, amaze your friends and relatives by getting a hard-boiled egg into a  small milk bottle without touching it. Be careful though, the results can be explosive, as Georgia Mills and Chris Smith found out...
small milk bottle without touching it. Be careful though, the results can be explosive, as Georgia Mills and Chris Smith found out...
Georgia - I'm going to get this egg which is quite a bit bigger than the rim of this milk bottle inside without squishing it.
Chris - Wow! Okay, I will bet you a tenner that you can't do it.
Georgia - Deal!
Chris - Go on then. How are we going to do it?
Georgia - Right. So, if you want to do this at home and win some money off your relatives, all you need is one of the old fashioned milk bottles with the sort of small glass rim at the top. This won't work in a plastic bottle. You need a hardboiled egg and some matches.
Chris - Okay, we now have a hardboiled peeled egg.
Georgia - Right. So, what I'm going to do is I'm going to put the egg down for a second and I'm going to light a match and then I'm going to drop it straight into this glass milk bottle then I'm going to put the egg on top and you'll see what happens.
Chris - Okay, right. So, let's light the match. Ready? [POP] Fantastic! What I will say for you Georgia is the egg is inside the bottle. It's not very egg-shaped anymore. I would say it's more like scrambled egg but it is in there.
Georgia - I didn't expect that to be honest. (laughing)
Chris - Okay, so what has happened? How did the egg miraculously get drawn straight into the bottle because literally, we put the egg on the top of the bottle and then there was almost like an explosion and it went straight in.
Georgia - Well, the key was in the matches and what the matches were doing in this milk bottle was heating up the air inside. When air gets hot, it expands. So, what happened there was that this hot air expanding was being pushed out of the bottle. There wasn't enough room for it there anymore. So, the pressure inside was temporarily higher. Now, when we put the egg on top and then the matches went out, the air started to cool down again which means it contracted. As there's now less air in the bottle than there was before, this means that there's really low pressure inside the bottle where the pressure outside of the bottle is the same as normal. So, it's much higher, pushing the egg right into the bottle and then creating this lovely omelette.
Chris - Where is this is sort of relevant because yes, I can understand the principle and it's a useful bit of physics, but is this relevant to everyday life?
Georgia - Outside of winning bets, it's really interesting to think about the amount of air pushing down on us all the time. each square meter has about the equivalent of a double decker bus of air pushing down on it all the time. the atmosphere is extremely heavy.
Chris - Well, I'll grant you this, Georgia. I think I did sort of lose the bet, but some of the egg did break apart when it exploded on its way and then has landed on the table. So, shall we call it a fiver?
Georgia - Sounds fair to me.

33:38 - A healthy chocolate bar?
A healthy chocolate bar?
with Dr Ivan Petyaev, Lycotec
Easter just wouldn't be Easter without consuming obscene amounts of chocolate. But for most of us, it's something we enjoy with a bit of a guilty conscience, worrying what it will do to our health and our waistlines. Wouldn't it be nice if this wasn't the case? Ginny Smith went to visit Lycotec's chocolate lab in Cambridge, where the founder of the company, Ivan Petyaev, explained how they are developing a range of chocolates that are actually beneficial to our health...
Ivan - Majority of people, one way or another, eat chocolate. It’s a treat most people love basically and we want not to change people to do something they don’t know, eat something different or a few people eat it. Whatever the reason, people do go for chocolates.
Ginny - Now, I've heard a lot about kind of health claims around chocolate particularly dark chocolate that it’s really good for you and all these different ways. What is it in chocolate that gives us those benefits?
Ivan - We identify a number of active molecules which is really responsible for a certain kind of benefit. It ranges from neurotransmitters which are present in the chocolate, two, caffeine, and in the last 20 years, main attention really focusing on new molecules they found which have been there all the time called polyphenols and flavanols. They're cousins or the same molecules which is in red wine, the same which is in the blueberry.
Ginny - What is it about polyphenols that are good for us? What do they actually do in the body?
Ivan - Polyphenols, it’s a type of molecules which is sitting on a surface of the cell membranes because it’s a little bit lipophilic. Also, it’s a bit water soluble.
Ginny - So, the molecule is both attracted to the fats around the outside of the cell, but it’s also soluble in blood.
Ivan - Yeah, absolutely right description because it’s sitting on a surface of a membrane exposed to the blood, to the plasma. This is a unique position because this is where all drama in the cells happening where the disease come. Oxidation starts from outside. Inflammation starts from outside. It means, you need to help cells to control this process.
Ginny - So, is it effectively protecting the cell from attackers in the blood?
Ivan - Exactly because when the cells get attacked by damaging factors, it would protect them and this has been described in many studies and we really like these properties.
Ginny - So, if chocolate is full of all this good stuff anyway, what makes your chocolate better?
Ivan - We’re not really bringing new chocolate. We’re not bringing new molecules. We have a technology which allow us to deliver these molecules in very compact form and make them working. Our technology designed to protect these flavanols in the chocolate because actually, it’s only 1% of flavanols get absorbed to the blood. if we learn how to protect flavanol from the damage during digestion process, we can increase delivery of the flavanols and we don’t need so much chocolate to consume.
Ginny - Does that mean you're saying that there is a huge amount of flavanols already in the chocolate but we just can't access them because by the time they’ve got to our stomach, most of it has degenerated and it’s not useful anymore?
Ivan - This is exactly what is happening. 99% get oxidised by acidity and bacteria. It means we have very little left to absorb.
Ginny - What exactly is it about the technology you’ve developed that prevents that oxidation process and keeps those flavanols available for our bodies to use?
Ivan - We know that flavanols are sitting within the chocolate crystals and we’ve developed a technology that we can code these crystals or some of these crystals with other active carotenoids. So pigments like lycopene from tomato, lutein from spinach or astaxanthin as well from algae. Basically, they're very similar to each other but main property of them, they're not just able to code the chocolate crystals but they have acid resistance. It mean this is what give us – use them as a protector of flavanols during digestion process. As a result of this coating, we can have a high level of delivery of these flavanols to the blood.
Ginny - And of course, those carotenoid molecules you mentioned are very beneficial as well. We’ve all been told to eat more greens and eat more tomatoes because they’ve got these pigments in them that are so good for us.
Ivan - This is correct. If you combine the benefit of digestion of these carotenoids together with higher increased level of flavanols, we’ll have a very nice energetic, health beneficial effect.
Ginny - And we really can eat chocolate without feeling guilty about it.
Ivan - Yes, you can eat it and enjoy it. You can try yourself the taste and we’ll see how it tastes.
Ginny - That will be fantastic! So, have you got some here that I could try?
Ivan - Yes, please do.
Ginny - Okay, so which version of your chocolate is this?
Ivan - It’s contained astaxanthin. It’s from algae, make basically food chain all pink: prawns eat them. Fish eat prawns and the creoles, and all of them, all of these marine species will have astaxanthin.
Ginny - So, it looks just like a normal square of dark chocolate. It’s lovely and shiny and it’s got a very pungent dark chocolatey smell. I'm going to try a piece now. You promise me this won't taste of algae.
Ivan - No.
Ginny - I'm not detecting any algae at the moment. It’s a lovely smooth dark chocolate. It’s delicious. You wouldn’t know that this was a health food certainly.
Ivan - Well, this is the point. We don’t want to change taste.

39:10 - Uncovering Easter Island's secrets
Uncovering Easter Island's secrets
with Professor Sue Hamilton, UCL Institute of Archaeology
To its inhabitants, it's Rapa Nui, but here in Europe we know it as Easter Island - one of the most isolated places in the world. It's a tiny dot in the Pacific Ocean, thousands of miles from the coast of Chile and home to some of these famous and mysterious statues in the world. Built around 800 years ago, these distinctive giant stone figures stand watch over the island's inhabitants and are still an important part of their ceremonial life today. But what do they mean and how on earth did the early islanders manage to make and move them? To find out, Kat Arney went to meet Sue Hamilton, director of the UCL Institute of Archaeology...
one of the most isolated places in the world. It's a tiny dot in the Pacific Ocean, thousands of miles from the coast of Chile and home to some of these famous and mysterious statues in the world. Built around 800 years ago, these distinctive giant stone figures stand watch over the island's inhabitants and are still an important part of their ceremonial life today. But what do they mean and how on earth did the early islanders manage to make and move them? To find out, Kat Arney went to meet Sue Hamilton, director of the UCL Institute of Archaeology...
Sue - It's about 5.5 hours across the Pacific and all you see is blue sea, blue sea, blue sea, and then you'll find a little triangular dot that's about 16 km by 23 and that is Rapa Nui. As you come down on it, you might actually think it was Scotland; it has no trees, lots of water. But when you get out, it's very hot. It's subtropical.
Kat - You called it Rapa Nui but obviously, we sometimes know it is Easter Island. Where did that name come from?
Sue - It was discovered on Easter day or discovered to Europeans on Easter day, 1722 by a Dutchman called Jacob Roggeveen. So, he went right across the Pacific to Chile and on his route back, he found Easter Island.
Kat - Where did these incredible statues come from? What do they look like? Who made them?
Sue - Well first of all, it is in Polynesia. So, it's a tradition of Polynesia to make effigies or statues which are put on ceremonial platforms. They're often considered to represent ancestors. But Easter Island is so remote that it developed a way of doing things of its own. It became quite extreme. So, the statues on Easter Island are far, far bigger than anything else in Polynesia, some up to 40 ft. high. What are they about? Nobody really knows for sure, but some of them are placed on ceremonial platforms which have burials around them and they're considered to represent the ancestors. The statues are also placed on these platforms near the sea. They face inland so they're also overseeing the landscape and the living. But some of them are never actually removed from where they were carved and they're carved out of a volcano known as Rano Raraku. Maybe 250 leave Rano Raraku and many, many more stay there, attached to the rock, set up around the rock, very much part of creating decoration of that place. So, we suggest that where the quarry is, is equally meaningful as the places that statues were taken to.
Kat - You've mentioned that these statues can be about 40 ft. high. You've shown me a picture where someone's standing next to one that's on its side on the ground. They are massive.
Sue - I think that's really important. The head of a statue is bigger than me. I'm not that big, but - I'm 5 ft. 2 for anyone who wants to know. Some of them have red hats on them. The hats are bigger than me. So, they very much oversee the island and when they were standing, they would've been a quite dramatic presence.
Kat - These statues have been built in a remote island in the middle of the Pacific Ocean. They're made of stone. They're absolutely enormous. How on Earth did they build them and move them?
Sue - So, they're carved in the quarry and that's quite accessible because you move along it as you carve it out. What's more dramatic is how you detach them from the rock. Some of them were detached, others are not detached. But volcano craters are very, very steep. So, as they detach, they're very vulnerable. They're kept on keels so they just detach by a little bit of stone until they're ready and then they would've been slid down the volcano, possibly on big wooden rollers from tree trunks.
Kat - What happens to them on their journey from the quarry to be setup by the sea?
Sue - In the quarry, the statues have no eyes. The position where the eyes would've been is marked out. Those that leave the quarry and we have found en route between the quarry and the sea, they have no eyes. But when they get to the sea and they're setup on platforms, big eye sockets are carved in their faces, and coral eyes are set in which have red or black pupils based on different rock materials they use. So, there's something to do with the animation of the rock, some ideas about when the statues are active.
Kat - They're kind of waking up when they get to the sea.
Sue - Yes. And of course, they're overlooking the island. So, those aggregate cultural activities and other activities on the island, you have the statues looking at them.
Kat - We think of these statues maybe something historic from the past - you know, that were made hundreds of years ago. Do they have any role on the island today?
Sue - They're completely central to the island. They're part of the belief systems of the island. So, I think that's very important because in Europe, we often look around monuments, as if there's something separate whereas these are very much understood as part of the world of the present. I'd also say, they're very important because they create tourism. There's not much that Easter Island can do to earn a living bar bare subsistence, but tourism is very important on the island. Of course, that is also important in terms of managing it. It's a tiny island. How do you manage a lot of tourists and how can you train those tourists to see that these things are meaningful in the present and should not be abused, but should be treated with care.

45:43 - Eggsperiment 4: Walking on eggshells
Eggsperiment 4: Walking on eggshells
with Georgia Mills, The Naked Scientists
Daring to stand on a carton of raw eggs; Chris Smith and Georgia Mills find out  about the impressive structural properties of an egg.
about the impressive structural properties of an egg.
Georgia - We're going to test the strength of eggs today and see if they together can support your weight. So, I've got a standard 6 eggs carton on the floor with 6 eggs in it. I haven't done anything to them. I've checked that there aren't any cracks in them and that's all. You're going to stand on them now.
Chris - This is this sort of cardboard carton that you'd get from the supermarket and there are 6 rather nice looking eggs in there and you want me to just stand with one foot to stand on that.
Georgia - Yes. It will be a shame if this goes wrong, but I have faith in the structural integrity of these eggs.
Chris - I have taken my shoe off. I'm putting my socked foot on top of the eggs in the egg box.
Georgia - And now, I want you to make sure your weight is evenly distributed across all of the 6 eggs.
Chris - Okay, I'm going to put my arm on you, just so I can just stay stable.
Georgia - Okay.
[CREAKING]
Chris - Okay, you wouldn't believe it and I'm very nervous doing this, but I'm standing on one leg, on one foot, on a box full of eggs. I'm genuinely doing that and they haven't broken.
Georgia - I'm just as surprised as you I think.
Chris - I'm very relieved. Honestly, I thought that my feet were going to go straight to them and I was going to have a very eggy sock.
Georgia - That's the amazing property of an egg. If we did this on their side, this wouldn't work at all. Do you want to try that Chris?
Chris - I'm not doing that, but let's just grab an egg. So, these really are genuine eggs. They're not hardboiled or anything. I can shake it. I can feel the sort of yolk and stuff slushing around inside. So, what's special about this that I can stand on it in that way?
Georgia - Eggs have a really good shape for this kind of compression. If you look at them, they're ovals. They're like an arch shape and we use this kind of shape in architecture all the time. you think about bridges, you think about domed ceilings. If you push on the top of an egg because it's such a narrow arch, the force is distributed all throughout the body of the egg. Meaning, it won't break. However, if you put a smaller force on the long edge of the egg, this actually squeezes in the inside of the egg as well and the eggs are really bad withstanding this kind of pressure.
Chris - What you're saying is, this egg is - because of its shape, when I apply force to the curved top of the egg, basically, I'm compressing all of the material in the egg in all directions. It's sort of transferring the force down all of the sides evenly whereas if I turn the egg on its side and I were to stand on that and I'm not going to do it - much to your disappointment, I won't be doing that. But if I were to stand on the side of the egg, then I would be bending the shell a bit and there wouldn't that transfer of load all over the egg. It would just basically bend the shell and it's not very strong when you make it stretch. It's going to bust open.
Georgia - Exactly, which is why when you crack an egg, you do it on the side. You'd never do it on the top.
Chris - Did eggs evolve to have this shape for that reason? Is that why the egg has got this property to stop the chicken literally cracking its own eggs?
Georgia - That's right. So, when a mother hen sits on her eggs, the last thing she wants to do is to squish all her babies. So, with these eggs pointing upright, it distributes her weight all around. But then when these tiny weak chickens need to get out, a small force on the side of the egg enables them to crack out of the egg.

49:19 - As useless as a chocolate teapot: part 2
As useless as a chocolate teapot: part 2
with Dave Ansell and Ben Valsler, Naked Scientists
Dave Ansell and Ben Valsler test out the teapot made entirely out of chocolate,  and find out if it can be used to brew a lovely cuppa.
and find out if it can be used to brew a lovely cuppa.
Ben - Welcome back to the Naked Scientists chocolate teapot tea party. Dave, you've made yourself quite an impressive looking chocolate teapot here. can you run through how it was made?
Dave - Well, I first got a quantity of chocolate, about 1.3 kg of it. I then got a bowl, filled it to the half full of chocolate and put a smaller bowl inside to make the gap to put tea in and then I made a tube of chocolate by getting a tube of grease proof paper, sort of plastering the chocolate and putting more grease proof paper on the outside, putting that on the fridge and setting it.
Ben - So, that's formed our spout. How have you actually attached the two together?
Dave - Basically, just got molten chocolate and used it as a kind of glue. It welds the two together really nicely.
Ben - Excellent! Who would've thought that chocolate would make a good welding material? I can see also that you have a handle. Although, knowing that there's over a kilo of chocolate there, I'm not sure I'd trust it.
Dave - No. I think the handle is there mostly for aesthetic purposes.
Ben - Well, I suppose it's not a tea pot if it doesn't have a handle and a spout. So, I guess the next thing to do is just find out if we can brew some tea.
Dave - Indeed. I've got some couple of Earl Grey teabags here, open the lid, put the teabags in.
Ben - There's plenty of space in there. We should get at least two cups of tea out of this. Well, water has just gone off the boil so let's pour it in and see what we can do. [POURING] Well, it certainly hasn't melted through yet. I can see that you've even made a chocolate lid. Now, that looks very thin compared to the rest of the teapot, Dave. How confident are you that this lid is going to hold up?
Dave - The lid is probably the lowest level of confidence I have I'm afraid Ben
Ben - Well, with all that steam condensing on the underside of it, we shall have to see. So now, I guess we just sit back for a few minutes and let the tea brew for maybe 3 or 4 minutes.
[MUSICAL INTERLUDE]
Ben - Well, it's already been 2 minutes since we poured the water in but unfortunately, we've had a bit of an incident with the lid. Dave, what's happening?
Dave - Well, hot air and steam rise. The heat is coming up then you got hot steam condensing on the bottom of this chocolate lid which is causing it to melt. So, the lid was very, very thin. It was rather an afterthought. If you want to make a chocolate teapot at home, make the lid more than 5 or 6 mm thick.
Ben - Okay, so the lid has melted through a bit, but the main bowl seems to still be very intact. How does it feel on the outside? Does it feel warm?
Dave - It still feels cold on the outside. So, it seems to be working quite well as an insulator. Obviously, the chocolate on the inside is insulating the chocolate on the outside. So, it's not melting. And now, for the moment of truth, we'll try and pour the tea out of the teapot.
Ben - I'm really, really impressed. The spout held firm. It does really look like tea as well. I expected it just to look like a chocolatey murky mess. Inside the bowl, it's all clearly melted but it's still pretty much bowl-shaped. It hasn't just become a puddle of chocolate.
Dave - I think what's going on is that although chocolate melts quite easily, when it's molten, it still got some structural integrity. It doesn't actually slump too quickly of its own accord unless you actually push it or it's trying to support its own weight like the lid was. Chocolate is made out of fat and fat is actually quite a good insulator. So, the molten chocolate was insulating the unmolten chocolate outside. So, the outside still stays solid and it didn't melt.
Ben - So, it's actually a layer of melted chocolate insulating the rest and this is why it didn't melt all the way through. It actually done a very good job.
Dave - Yeah. Chocolate is obviously better than you'd expect from making teapots out of.
Ben - I guess we better try the tea and see if it's any good. Cheers Dave!
Dave - Cheers! Ben.
Ben - That's actually not too bad, you know. It does taste a lot sweeter than I'd usually have my tea, but it's still hot, it's perfectly palatable and I think we've proved actually that you can make a teapot out of chocolate.
- Previous Niacin: Chemistry in its element
- Next Yeast: Rising from the bread










Comments
Add a comment