Periodic Table: 150 Au Years
This week we’re celebrating 150 years of the Periodic Table - we’ll find out how scientists uncovered the elements in the first place and what other mysterious materials may be waiting to be discovered. Plus a way to power up the body’s own morphine-like chemicals, how microbes are gluing microplastics back together in the ocean, and post-Valentine’s Day, some dating do’s and dating dont’s to bear in mind for next year!
In this episode

Morphing morphine
with Peter McNaughton, King's College London
Millions of people are affected by chronic pain; despite this, we're not good at treating it. But a discovery by scientists in France that's enabled them to power-up the pain-killing effect of the body's natural morphine-like chemicals, and target the effect to where in the body it's needed, means we might be about to become much better at - as the old saying goes - hitting pain where it hurts. Peter McNaughton is a pain specialist at King’s College London. He wasn't involved in the new study but he agreed to take a look at the paper for us and he explained to Chris Smith how it works...
Peter - What this group have set out to do is to find out an alternative to the opiate class of drugs: morphine heroin etc. Now these drugs are fantastic painkillers. They work really well, but they have a number of huge downsides. They cause addiction; they cause respiratory depression, which can cause people to die in their sleep. And these adverse side effects are all caused by penetration of morphine etc. into the central nervous system.
Chris - And what you're saying is it's because they get into the the brain and spinal cord - the central nervous system - that we get that downside? If we could stop that that wouldn't happen?
Peter- Exactly. And that's what they've tried to do. They've invented a - it's not really a new drug - it's a new twist on an old theme. What they've invented is a drug which doesn't enter the central nervous system, and therefore, shouldn't have all these nasty effects we talked about a moment ago.
Chris - How does it actually do that?
Peter - Well, they've done it by using what's really an old idea which came from Hans Costalitz who was working up in Aberdeen. Costalitz found that there is an endogenous opiate produced within the body. And he really discovered this just with a very simple piece of reasoning, which is that drugs of the opiate class - heroin morphine etc - are so potent that they must be latching onto a system which exists within the body already, and he isolated some peptides which are called the encephalins.
Chris - So these are compounds which are naturally present in the body and they activate the nerve pathways that morphine - when we inject that - does, and that's how they achieve their pain killing effect? The difference being that you can make those compounds yourself?
Peter - Exactly. These are the "endogenous opioids" as they're called. They have a powerful analgesic effect. You can see why this is important from an evolutionary point of view. If you're in a highly stressful situation, you're being chased by a bear or something like that, there's no point in saying "oh, ow I'll stop - the bear's biting my ankle!" - You've got to get away, otherwise you're going to die. And therefore, in stressful situations, the release of these endogenous opiates give a tremendous analgesic effect, which means effectively that you can escape by a life threatening situation.
Chris - And the group are exploiting, what, those compounds and the way in which they work in order to produce this new way of doing pain relief?
Peter - Exactly. The encephalins are peptides, and these are compounds that are metabolised very rapidly in the body so they produce an analgesia which is very short-lasting. So from that point of view there's not much point in the injecting encephalins into people's bloodstreams; the analgesic effect will be gone in a couple of minutes. What this group have done is they've linked up encephalin with a lipid - a natural body lipid - called squalene. They found that that makes the lifetime of the encephalin very much longer. And the other advantage of squalene is many squalene groups sort of make a lipid "droplet", which is like a hedgehog: it's got the encephalins sticking out, and this produces a very long-lived form of encephalin, even longer lived than morphine.
Chris - So if you inject it, it will circulate for a long time; it can bind onto all the right nerve endings and block the transmission of pain, but it doesn't seem to get into the brain and spinal cord and cause the adverse effects of morphine?
Peter - Exactly. And in fact, one of the things they've shown, which is even more interesting, is this circulating lipid droplet, with the encephalin peptides attached, seems to be targeted specifically to areas of pain. Now it's been known that when you have local inflammation that causes pain, that makes the blood vessels more leaky, and it's at those specific spots where the blood vessels are leaky that the compound is able to leak out and have its analgesic effect.
Chris - How have they tested it to prove that it is actually as good as that?
Peter - They've attached a fluorescent compound to their encephalin-squalene drug. And they've done whole-body imaging of animals, and the drug accumulates in areas where there's a localised inflammation that's causing pain and it doesn't accumulate in other places.
Chris - And can they also prove though that, in those animals, they're not feeling pain because they've injected this?
Peter - Well, not in exactly the same animals, because the animals that are being imaged are unconscious, but parallel groups of animals they've been able to find that pain is very very much reduced by the injection of this encephalin-squalene complex.
Chris - Will it work in a human?
Peter - Well it should do. Of course, we don't know that. These are animal experiments, but I can't see any reason why it wouldn't work in a human. I just have to point out a downside of this method of getting the drug onboard to the patient that it has to be injected intravenously. And if you're treating a soldier in a battlefield situation that's something that's going to be rather difficult to do...
HPV: 10 years of the vaccine
with Margaret Stanley, Cambridge University
Human Papilloma Virus, or HPV, is the name for a group of viruses that commonly cause verrucas, warts, genital warts and cervical cancer. And they are very common. The infection usually resolves itself, but in some cases it leads to damage to the DNA in the infected cells, which can result in cancer. This is why we have a screening programme to look for these changes and try to pick up the disease early before it can spread. Just over ten years ago, a vaccine against the highest risk forms of the virus was introduced; in Australia boys and girls received it, but in other places it was given just to girls. The UK was one of them. But 2019 will see it also being offered now to boys, as well as changes to the way we screen for HPV infection. Margaret Stanley helped to develop the HPV vaccine at Cambridge University, and she spoke with Chris Smith. First up, Chris asked Margaret what 10 years of data shows about the vaccine; does it work?
Margaret - Well I can give you the information from the UK and the UK has had a spectacularly successful vaccination programme, and we're seeing the results of that now and I can quote what's happened in Scotland. The girls who were vaccinated in 2008 in Scotland aged 13/14, by the time they were 20, in 2015/16, came up for their first cervical cancer smear - because in Scotland, at that time, you were getting smears at 20 - so we know what proportion of those girls we expected to have the pre-cancer - that's what the cancer screening programme's about - and what proportion did.
In fact there was a reduction of eighty six per cent in the pre-cancers in that group of vaccinated girls. That's spectacular. Part of the reason why is in that young group of girls those two very high risk viruses that the vaccine targets are the ones that cause most of the pre-cancers and the cancers. So the vaccine is stunningly successful. Same data from Australia; same data from Denmark. Wherever you've got a high vaccine coverage - now I want to emphasise that: we've been really successful in the UK because almost 90 per cent of our 13 year olds are vaccinated, whereas in other countries where it's much lower you're not getting the result.
Chris - Yes so the data you quoted is for people who have been vaccinated against people who would have been otherwise identical to that group and haven't. So if we look at those people who haven't, roughly what number of cervical cancers are we seeing or pre-cancers are we seeing in those people normally?
Margaret - The pre cancers sort of peak in the 20s, and you expect to see about two per hundred individuals for the pre-cancers. Now not all those pre-cancers go on to cancer, but those two individuals will have to be treated. So what we're doing is taking out almost all of those pre-cancers, losing that treatment. If you haven't been vaccinated, however, you've got to be screened, and you still ought to be screened even though you're vaccinated, because not all of the HPVs that cause cancer are targeted by the vaccines, but your risk is massively reduced.
Chris - What was the rationale, was it just cost, for only going for the girls and not vaccinating the boys in countries like the UK?
Margaret - Well it's because genital HPV is a sexually transmitted infection, and if you immunise one gender then you protect the other gender. And so decisions about who to vaccinate, when and how many, is based on modelling and on health economic policy. So the models said well if you can get up to 85-90 per cent of girls vaccinated, we won't need to vaccinate the boys. But it turns out that men get cancer of the throat, which is caused by HPV and they're four times more likely to get it than girls.
Chris - And the vaccine will stop that?
Margaret - Well I have to be very careful; I have no data from trials that says that the vaccine will stop the cancer. I can tell you that the vaccinated people lose their infection in the mouth, so it prevents the infection in the mouth. And you know I'm a simple soul, if you don't have the infection you won't get the cancer.
Chris - Now you mentioned that the vaccine represents some of the highest risk forms of the virus, but it doesn't cover all of them. So we're still having to do cervical screening. Why can't we add the missing viruses into the vaccine and if it's so successful?
Margaret - Well there’s a second generation vaccine, which is commercially available, and that's the one actually which is now being delivered in Australia and the USA, but the decision hasn't been made yet in the UK as to whether to put that into our programme.
Chris - And that does represent these other forms that are missing at the moment?
Margaret - Well it represents, for the sort of global take on this is the vaccine that targets the two high risk viruses, which is what we currently use, will get rid of 70 per cent of cervical cancers worldwide. You put this other one in which has another four high risk HPVs, you'll get rid of up to 90 per cent of cervix cancers worldwide; but you'll still have a little rump of cancers which are not covered by the vaccine.
Chris - So we still need to screen and that brings me on to my only point which is can we use the learning that's gone with the study of human papillomaviruses together with much better diagnostic techniques that we now have where we can screen for DNA and things like that now, rather than just having to look at cells under microscopes. Can we use this knowledge to do a better or better screening job?
Margaret - Yes the screening programme in England and the rest of the UK is going to change in 2019. From the first analysis being looking down the microscope itself to in fact detecting DNAs of these high risk viruses. You'll still come for your smear at 25, but instead of that smear being looked at down the microscope first, it will be tested for DNA. If it's DNA positive for the virus, then the screener will have a look at those cells, and if the cells are normal then you'll go back into the programme and be asked to come back and have another screen in about a year's time to make sure you've got rid of the virus.
Chris - Crucially if it's negative you need go no further at that time. So it’s going to save loads of money and time.
Margaret - It's not only that, it's going to save all the hassle of going for a smear, and it's a much easier and much more convenient. More importantly it's more sensitive. The trouble with cervical screening is it's a wonderful public health intervention but although it's very specific, it's not very sensitive.

The drum around the Earth
with Martin Archer, Queen Mary University London
The magnetosphere is a protective layer that surrounds the planet. It's formed by charged particles rushing in from the Sun and interacting with our magnetic field. Forty-five years ago a theory was set out suggesting that when it’s hit by a surge of radiation from the Sun, the whole magnetosphere shakes, like the surface of a drum. This, the theory says, produces electromagnetic radiation that can have consequences for systems on Earth, including our technology. This week scientists proved it really does happen. Adam Murphy has the story…
[NOISE]
Adam - The sound you just heard is streams of charged particles travelling at 400 kilometres per second from the Sun hitting our magnetosphere. The magnetosphere is a layer around the planet formed when particles from the Sun meet our magnetic field. It helps to protect our planet and our technology from the harshest effects of these solar streams. And just like striking a drum, when streams from the sun hit this magnetosphere they make ripples in it. Martin Archer is a planetary physicist from Queen Mary University London who's been looking into a 45 year old theory about the Earth's largest drum.
Martin - What we found is some of those ripples which will head towards the northern and southern Poles will get reflected back and you'll end up getting a pattern called a standing wave that's very much like how you get patterns on the surface of a drum and which gives them their notes. So that's what we found about the magnetosphere in this particular study.
Adam - A standing wave is a wave that looks like it's standing still; like a guitar string, or in this case a drum.
Martin - The way that we drew this picture together was by using five different satellites from NASA's THEMIS mission that happened to be in the right place at the right time and essentially allowed us to see the thing hit the Earth's magnetosphere, see the boundary move in response and hear the sounds within our magnetic environment that were caused by it. So it was piecing together those observations that allowed us to finally make this discovery.
Adam - But since particles from the sun are always hitting the Earth how do you isolate one single solar slam.
Martin - It's a complicated problem being able to show direct causality from one thing to another, often because you've got lots of things happening at the same time, all at once. Lots of different processes that can lead to something quite complicated. So that's why we've picked a very specific sort of impulse it was a very strong jet of plasma hitting into our magnetosphere with nothing really very much either side of it happening. So that means we could be very clear about that it was definitely this and not something else going on that was that was causing the effects that we saw.
Adam - And understanding this, it isn't just pie in the sky stuff.
Martin - So the consequences of this are things that we still need to do a bit more work into. But we certainly know that these standing waves do penetrate within to our magnetosphere quite deeply and they're a source of ultra low frequency waves.
Adam - So when these streams from the sun hit the magnetosphere they cause all the charged particles there to wiggle which creates these waves in the magnetosphere itself. And these helped create ultra low frequency waves which may have profound effects on our technology.
Martin - They can accelerate electrons in the radiation belts up to energies that can damage satellites for instance, and there are ideas as well that these waves might affect the Aurora and they can actually heat the top of the atmosphere. So there's lots of different ways in which they could have consequences mainly on our technology. But as I say because we've only just discovered these in the observations now, we've got a long road ahead of us to do further research into really understanding the actual implications.

18:33 - New wave of nanoplastics research
New wave of nanoplastics research
with Tony Gutierrez, Heriot-Watt University
Nanoplastics and microplastics are small particles that form when larger pieces of plastic break apart. And this is happening extensively in the ocean, although we don’t yet know what the consequences might be. One outcome is that sticky polymers produced by microorganisms can glue these small plastic particles back together so they form agglomerations that other species mistake for food. Mariana Campos spoke to Tony Gutierrez who’s been studying the effect at Heriot-Watt University in Edinburgh...
Tony - We were interested to understand what happens to nanoplastics in the marine environment. And we observed that like with microplastics they form into agglomerations with biopolymers. These are gloopy, sticky substances that are produced and released by bacteria and microscopic algae or phytoplankton in the ocean.
Mariana - How did you get to the results that you got?
Tony - What we did was we incubated natural seawater with some nanoplastic particles. Within hours we observed the formation of particles and these particles grew in size. And we could see these nanoplastic particles embedded within a web of polymers, and what we wanted to find out after this was what type of polymers could be enveloping these plastics. So we extracted polymers from a particular species of marine bacteria that produces a lot of these polymers, and we incubated them with the plastic particles and indeed we observed the very quick formation of plastics embedded within this web of polymer.
Mariana - Are the agglomerates dangerous?
Tony - We don't know what their impacts could be. One thing we know is that what were invisible nanoplastics have become visible by the formation of these agglomerations. So what this could lead to is these particles which are now visible could be mistaken or seen as a food source and ingesting the plastics that are within them. We don't know what the impacts could be. We do have some data that suggests that there are no toxicological effects, but we still need to do more experimentation to understand that.
Mariana - Have you got other experiments planned for the future?
Tony - Yes so one thing we want to look into that I'm very interested in is, in the formation of these plastic agglomerations, does it have an effect in terms of the degradeability of the plastic particles. So could these agglomerations be a hot zone in the marine environment where the plastics could be broken down or degraded faster by the microorganisms that are associated with these agglomerations. So that's certainly something I'm very interested in doing. What I'm explaining is something that's occurring naturally in the environment. How to enhance that, to get rid of plastics, that's something that really deserves a lot of thinking. I think we still need a lot more information. But one thing at the end of the day really is in order to reduce the concentrations of nanoplastics and microplastics in the environment we just simply need to reduce the entry of plastics into the oceans. So implementation of policies and so forth that really reduce the amount of plastics that go into the environment.

Dating do's and dont's
with Viren Swami, Anglia Ruskin University
We’ve just kissed goodbye to Valentine’s Day for another year. And if it didn’t work out the way you’d planned this time around, perhaps you need to alter your approach? To set you on the right track, we sent Katie Haylor to see social psychologist and dating expert Viren Swami, at Anglia Ruskin University, to hear his top tips for dating “do’s” and dating “don’ts”, starting with that old chestnut, playing hard to get...
Viren - In general playing hard to get doesn't work because it contravenes what's called the theory of reciprocity, that we like people who like us, and dislike people who dislike us. So if you show any form of dislike for someone like playing hard to get, the theory is you they will dislike you in return. Now it's slightly more complex than that. They may want you a bit more, but they may not like you very much when they get you. You want to give the impression that you like that person because liking sparks liking in return. The best suggestion is to play selectively hard to get, to show the other person that you like them as an individual, but it's actually very difficult for other people to get you.
Katie - Another piece of advice is to date someone who is very different from you. This idea of opposites attracting. Can we bring out qualities in each other? What do you make of this?
Viren - There is zero psychological evidence to suggest that opposites ever attract. A very very old idea in fact about 60 percent of general public believe that your ideal partner is someone who has the opposite qualities to yourself. All the studies from psychology suggest that more like two people are in terms of their demographics, in terms of their values, in terms of the maturity, in terms of their life goals, the more likely they are to get along.
Katie - But that doesn't necessarily mean you have to be very very similar, does it?
Viren - In the short term no. So say on a first date you want to be with someone who is relatively similar to you and perceived similarity is probably more important, so you both think that you are relatively similar. If you end up having a relationship, most couples tend to engage in something called attitude alignment, if they they're invested in the relationship. If for example you and I disagree on Brexit, and we're in a relationship, we'll find some common ground or minimize the things we disagree on.
Katie - That's some do. Dont's - a big one I've come across looking online is talking about your exes. This has got to be a no no, right?
Viren - Absolutely. On the first date you don't want to put all your stuff out there. It shows that you're still stuck in the past, that you're not really ready to move on and have a positive relationship with someone else. You might also want to think about why it is you're talking about your ex on a first date. Are you just looking for someone to listen? In which case you might want to talk to your friends rather than a potential partner.
Katie - Another don't - spaghetti bolognese, pizza, are there really any hard and fast rules about food that you should eat when you're on a date?
Viren - The thing with rules is I hate rules. Human beings are so complex and we're just so complicated that you never know how someone's going to react. Someone else might find your sloppiness, your messiness with spaghetti bolognese really cute. Another person might find it really disgusting so it's really difficult to know. The important thing is if you're planning a first date to both decide together where you'd like to go, and if that's a messy food place then hopefully you'll both enjoy it.
Katie - Obviously this is quite lighthearted, but there is a bit more of a sinister side to dating advice. I've come across a concept called "negging"?
Viren - So negging is an idea that comes from pick up artistry. Pick up artistry more generally is this idea that's typically sold to men that they can program women to behave in particular ways as a result of particular use of language or particular behaviours that they do. Scientifically there's no evidence to support it, it's considered pseudoscience. One version of that is negging, pay a woman a backhanded compliment and that will make that woman want to come to you even more.
Katie - So is this something like "Wow you're a bit cleverer than I thought you'd be by looking at your picture"?
Viren - More along the lines of "you look really horrible but at least you're a nice person". From a scientific point of view it contravenes the theory of reciprocity, if you're horrible someone they're not going to like you in return. You want to show you like that person. I think a lot of pick up artistry is based on misogyny. This idea that you can program women to listen to men's use of language and respond in return. It's all nonsense.
Katie - Is there one piece of advice you'd give people to have a nice date?
Viren - Just be a nice human being, be a decent person and treat other people with respect. There is a good reason why this is important and it's based on the halo effect. This idea that we transfer perceptions of a person's characteristics onto some other characteristics. So if for example you're perceived as being a nice human being, sometimes that also transfers into perceptions of your physical attractiveness. In very simple terms if you're a nice person in the long term you also get perceived as being more physically attractive than you actually are. Second reason why it's really good to be nice is it just makes everything better. Particularly, if you're going on a first date it can be really stressful. Just being nice makes everyone feel better about themselves.
There is another thing you can do which is to get your partner to hold a warm cup of tea or coffee, because the idea is that they will transfer the warmth that they're feeling into your personality and essentially they will come to think of you as being warmer than you actually are.
Katie - That's genius!

27:57 - What's an element?
What's an element?
with Jenny Gracie and Adam Murphy
2019 is the 150th birthday of the chemist's best friend, the periodic table of elements. But what exactly is an element? Jenny Gracie and Adam Murphy have the quick fire science on the subject...
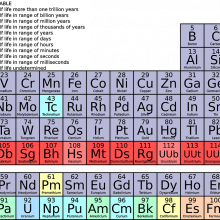
Jenny - Do you remember that giant wall chart that stared at you during science class at school? Like a game of tetris - lines of different coloured blocks slotted into a grid, with a few more lines of blocks below. This chart actually shows all the building blocks, or elements, that make up everything on Earth, from the microphone I’m speaking into, to the ears that you are listening with. This special table tells scientists how these elemental building blocks behave.
Adam - But what actually is an element? Each element is made up of one type of atom, and each is given a letter, like H for hydrogen, or O for Oxygen. Atoms have 3 basic building blocks themselves - protons, neutrons and electrons. In the centre, the nucleus, you’ll find the positively charged protons and neutral neutrons. So to balance the charge, whizzing around the nucleus are layers of negatively charged electrons. What defines one element from another - is how many protons it’s got (called the atomic number). Hydrogen has 1, oxygen has 8, and you’ll see this number next to each symbol on the periodic table.
Jenny - Now the vertical columns in the table are called groups, and these can be thought of as element families. Each group has the same number of electrons in its outer layer, which tell us how it might react with other elements. The first group all have 1 outer electron, and these often react violently with water, group 2 have 2 outer electrons and the pattern pretty much continues across the table. It is these outer electrons that form bonds with other elements, and also what determines the shared properties of a group.
Adam - Some elements like iron, copper and gold have been used by ancient civilisations for thousands of years. To date, there have been 118 elements discovered. To mark it is 150th birthday, we’re taking a tour of the periodic table, asking; how we tell one element from another; are we using elements sustainability, and what might the future hold for life’s essential building blocks.
30:07 - Periods and groups
Periods and groups
with Peter Wothers, Cambridge University
The periodic table is essential for all chemists and an important tool for predicting how the elements will react. But how were the elements we know and love actually discovered in the first place? And who put the table together? Chris Smith spoke with chemist Peter Wothers from the University of Cambridge, asking firstly, where did it come from?
Peter - Well actually it took quite a while to develop. I mean it didn't just appear in one go, and actually a number of people independently discovered this all around the same time actually in the eighteen sixties, but there was a long time leading up to that different forms began to emerge in a sense.
Chris - How did people realise that there was this relationship between the elements that we see around us and that we could arrange them into a table like that?
Peter - Well people were beginning to look for some sort of structure in the elements and the similarities that they had way before the periodic table itself came about. So for instance there were certain elements that were recognised as having very similar properties. Some of these for instance were these so-called ‘alkaline Earths’ and actually even before they were identified as elements in our modern sense, their compounds were recognised as having very similar properties. And eventually when the metals were discovered they were called the ‘alkaline Earth metals’ because again they all shared similar chemical properties. Similarly the ‘alkali metals’ as you heard they all fizz and react with water very violently. So that whole group were recognised as something that were very similar to each other. And so then people began to look for ways that these similar elements might be put together in an arrangement.
Chris - If one looks at the periodic table, so far over to the left, that's what we call group 1 and they are the elements, the alkali metals you've been mentioning, that fizz when we drop them in water. What's the difference between the ones right at the top left and as you go down that block on the far left hand side. How do they change physically the element?
Peter - Well they change in a number of ways I mean that the most basic thing is actually that they get heavier as you go down the group. So the individual atoms are increasing mass. In fact lithium, sodium, potassium are all light enough that they would float on water but actually rubidium and caesium would sink in water, but then they would react very violently in it. But there's also more subtle properties as well. So for instance how easy it is to remove that outermost electron that all of those elements share becomes easier and easier as you go down the periodic table. That means in a sense that those metals become more reactive.
Chris - So the ones on the far left are all metals. As we move across to the right you get into the group next to group one the alkali metals and they're a bit less reactive aren't they. Things like magnesium but they behave in a sort of similar way and then as you go across what changes?
Peter - So as you're moving across the periodic table what is happening is you're adding one extra proton in the heart of the nucleus. And so this in a sense will hold all of the electrons sort of more tightly in, and so then it's harder to remove the two electrons from the next group and actually by the time you've got all the way across the right hand side it's very very difficult to remove any of those electrons. And is actually their properties the more that they would accept electrons they would gain electrons and become negatively charged ions in some cases.
Chris - I remember my chemistry teacher putting a sort of ruler across the periodic table on the far right hand side and saying everything to the left of this is a metal and the things to the right of this ruler are non-metals and there are far more metallic things that behaves as metals, than there are non metals. Why is that?
Peter - That's quite difficult actually it's all to do with the number of electrons that each atom has and how they can form bonds. And actually you only get molecules within the elements for the right hand side of the periodic table, when for instance you get two oxygen atoms forming an oxygen molecule. But for the other ones you get larger structures with fewer electrons trying to form weaker bonds between all those. That's a typically a metal really with sort of these more what we call them delocalised electrons, electrons that can move around more easily throughout the whole structure.
Chris - Now when Mendeleev put his table together it didn't look like the one we have today. He actually left gaps. Was that because he realised there was this this sort of periodic behaviour? Things did follow a pattern and a sequence of behaviour and so he knew that even though we had discovered something yet there had to be something that would fit with that sort of position in the table. Is that where those gaps originated?
Peter - Well of course I mean Mendeleev wasn't the first to draw up his periodic table and others before him also left gaps for elements that were yet to be discovered. I mean the real difference with Mendeleev and his periodic table is actually that he very accurately predicted the properties of those missing elements and their compounds. And when they became discovered that's what really drew people's attention to his system.
Chris - When was the sort of golden era for discovering elements? When was the gold rush that loads and loads of them began to appear and fill in the table?
Peter - Well to fill in the table you had to have a table but they do come in sort of spurts. But I mean one of the key things actually in the late 1850s and early 1860s was the discovery of spectroscopy. So that revolutionised the discovery, because remaining elements at that point were very rare in a sense, so hard to find. And so you needed a very, very sensitive technique and that was the sort of flame test idea that allowed some elements to be discovered all in one go.
Chris - I'm glad you brought that up because we're going to hear about the pioneer of that, Robert Bunsen next. But sitting in front of you you have something exciting that you wouldn't reveal to me earlier. What's that?
Peter - So this is actually a copy of the very first periodic table which is seven years before Mendeleev's version. You say that Mendeleev's version looks very different from the modern version. Well this one looks completely different. This one was designed to be wrapped around a cylinder and actually it has a continuum of the elements arranged by their weight as Mendeleev also arranged them of course. And on this version the elements with similar properties also align vertically in groups.
Chris -Where did you get that?
Peter - So this one is one that is owned by St Catherine's College and will be on display there in March and April this year.
Chris - In line with the 150th anniversary of the periodic table as a concept?
Peter - That's right. We will be showing this very first form of the periodic table. And indeed a number of other versions including Mendeleev's first versions as well.
Telling elements apart
with Leesa Ferguson, Anglia Ruskin University
150 years ago the average scientists’ laboratory looked very different to what we have today. So how exactly did scientists test what each element was, beyond just looking at them? Katie Haylor went to Anglia Ruskin University to find out, with the help of forensic scientist Leesa Ferguson...
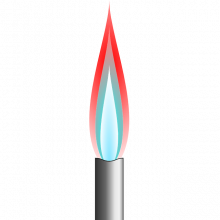
Leesa - Well they typically use flame tests. So what they would do is put individual elements into a flame and the flames would give characteristic colours and that's how they identified different elements.
Katie - Remember those Bunsen burners from school science class. You've got a gas inlet and a valve to control the airflow and these burned together to produce a clean flame. Crucial for doing a flame test. And this was something that chemist Robert Bunsen back in the 1950s or so was particularly interested in.
Leesa - He originally started work on flame tests but he found that the flames that he was using sometimes produced soot and weren't necessarily clean flames. And sometimes interfered with the actual colours of the elements he was trying to get. And so what he developed was the Bunsen burner because that gave you a flame that was non luminescent and therefore did not interfere with the colours that the actual elements produced when they were put in the flames.
Katie - So what's actually going on in a flame test? How does burning an element tell you what it is?
Leesa - So typically what is happening is that as the actual element is heated up, the electrons in the element get excited and they go from what we term a ground state to a higher energy state. So when they're in that higher energy state they then drop back down to the ground state and as they do that they emit wavelengths of light, coloured light, that is characteristic of the element.
Katie - Almost like jumps, jumping up and down?
Leesa -It is indeed, a little bit like jumping up and down a ladder. On a ladder you can step on one rung to the next rung, but you can't step in between the rungs. So that's a little bit like the energy levels.
Katie - And the distance between the rungs is separate for each element?
Leesa - It is indeed. And so when they actually emit light and it drops back down to the ground state, that would be characteristic of the element.
Katie - Well that's the theory down now let's put it into practice. Once me and Lisa were kitted out with lab coats safety specs and gloves, we got stuck in. We've got a Bunsen burner some matches, just putting the gas on now, let's get this Bunsen burner lit. Now what are you holding, and what are you about to do?
Leesa - It's just an implement that's going to enable us to actually put the element that we're looking at, in this case copper. into the flame.
Katie - But you're going to clean it to make sure we don't get any contamination?
Leesa - Yes I'm initially going to clean it with hydrochloric acid and then put the implement into the flame. So it just makes sure that any contaminants that may be present are no longer there.
Katie - So you're not doing a flame test for the wrong thing?
Leesa - Absolutely yeah.
Katie - So you've got some copper pieces. I guess the idea is to attach it to this implement and then see what happens when you put it in the flame. Oh that does look a bit green or a different kind of blue.
Leesa -You can see a characteristic sort of green flame that you do actually get with copper.
Katie - Is this how fireworks work? Because you get lots of different colours in fireworks and there's lots of different metals involved.
Leesa -Yes, fireworks typically contain lots of different metals, when they're heated up they actually emit energy of different wavelengths which is the different colours that you actually see.
Katie - Flame test in action, very expensive flame test. So what colours would you typically see with other elements?
Leesa -So with sodium you typically get quite an intense yellow colour, with potassium a lilac colour with lithium it tends to be a red colour. So there's some of your basic elements. Bunsen and Kirchhoff by using flame test coupled with a spectroscope actually identified two new elements, caesium and rubidium.
Katie - The spectroscope that Leesa mentioned contained a prism inside Think Pink Floyd's Dark Side Of The Moon album cover which splits light into all its coloured components. They found that the lines that appeared within the spectrum corresponded to the light being emitted by the elements. The wavelengths of light that each element emitted was unique, and this is why a spectrum is often thought of as an elements fingerprint. So are scientists using these same techniques to identify elements now?
Leesa - We don't typically use flame tests, we've got more advanced techniques that we tend to use to try and identify elements present in samples. Typical technique that we would use is something called inductively coupled plasma optical emission spectroscopy or ICP-OES.
Katie - I can see why it has an acronym carry on.
Leesa - And so basically it works on the same sort of premise really. You've got, let's say a sample of pure of element, the actual instrument would suck up the sample into something called a plasma. It has a really high temperature. So when the sample is in the plasma the actual solvent its in evaporates off, and the actual elements there are atomised. And what happens is that the electrons in the elements again get excited from a ground state up to a higher energy state and it's different for each element. And as they fall back down to the ground state the elements they emit a light of a specific wavelength.
Katie - As it turns out it's not just flames or even plasma that can be used to excite electrons in spectroscopy. Other wavelengths or parts of the electromagnetic spectrum can get involved.
Leesa - So different parts of the electromagnetic spectrum have different energies associated with them. So something like X-rays is very energetic, so it's so energetic that actually when you irradiate a sample with X-rays, in a technique like X-ray fluorescence, what that causes is actually electrons from the core, the inner electrons, one of those to be ejected actually out of the sample you're looking at. So because this electron is ejected from the core, one of the outer electrons drops down and fills that unoccupied space and because the electron has gone from a higher energy outer orbital to a lower energy inner orbital, it gives off an X-ray that's characteristic of the actual element you're looking at.

42:22 - Safeguarding element supplies
Safeguarding element supplies
with Lizzy Ratcliffe, Royal Society of Chemistry
As a society we all know that we need to change our habits to keep things like energy consumption sustainable. But have you ever thought about element sustainability? Could we run out of them in the future? Chris Smith spoke to Lizzy Ratcliffe from the Royal Society of Chemistry....
Lizzy - We're very aware of sustainability as a thing. In terms of things like plastics or energy or fish stocks or sustainable housing. But we don't think about is certain areas of the periodic table that we might not have heard of. So I don't know if you think about whether yttrium is sustainable, but actually it's not. All the naturally occurring elements you have to dig them out of the ground, they’re in mines and once they're gone they're gone. And a lot of them we're using in our daily lives and we don't even know it. So many of the rare and precious metals are present in our electronic devices. So in our mobile phones, Xboxes, Smart TVs, in pretty much everything we use so everything we need for modern tech comes from this part of the periodic table where there's not a lot there.
Chris - So the elements are not all made equally in terms of abundance? Some of them we really like but there's not much of them on Earth.
Lizzy - Yeah exactly. So there's plenty of aluminium, plenty of iron. We're not going to run out of pots and pans anytime soon. But indium some people think it might run out in the next 50 years, some people think it might run out sooner.
Chris - When you say run out, does that mean literally. I've got an old mobile phone sitting in front of me here I understand there's something like 30 key elements go into just making one of these. But when you say run out does that mean that basically we've made so many mobile phones that all of these rare elements are in all these devices and in active service. So we haven't got enough of the element left to dig up.
Lizzy - The thing is that most of us aren't really recycling our mobile phones and they're not made in such a way that they're easy to recycle. So the concern is that they could all end up in landfill. So at the moment we're digging out of the ground, we're using them, but we're not putting them back into the system. So there really needs to be a circular economy where we can plow the elements back in. Otherwise yes, they will run out. If they end up in landfill we won't have them, and not just for technology, but for other things as well. So a lot of the elements we're using in our phones they're also used for aircraft engines, drill bits, hearing aids, pacemakers, they all have all these uses and we don't even know what some of them might be needed for in the future, while we're squandering them on our mobile phones.
Chris - This old phone it's very old it's a clamshell type of phone, that says how old this is, but that there will be a sort of a suite of materials in there which will have been used to manufacture it. Are they easy to scavenge back though? So if I took it upon myself to recycle this, how easy is it to get things like indium out of that so that you could reuse it?
Lizzy - It's not particularly easy at the moment. And part of that is because manufacturers are making them in such a way that they're not easy to dismantle. So some manufacturers make them with custom screws or special glue so you can't just break them apart. So that's a big problem. And even when you do break them apart getting the elements out isn't that easy. But if we start thinking about that at the manufacturing stage it could get a lot easier.
Chris - But I'm surprised given that we've learned our lesson in so many ways, so many times in the past, about things like this with squandering resources and using them sensibly and making them recyclable. Plastics, we talked about earlier in the program, classic example. Why are we not doing this with the elements in these?
Lizzy - Well this being the International Year of the Periodic Table is a great time to start thinking about that. It's just not made its way to the public consciousness yet. I wasn't aware until I start doing this research as part of our celebrations. I wasn't aware of how many rare elements are in my phone or in any of my devices. It's just not something that we think about yet.
Chris - But if I take it upon myself to have responsibility and recycle my milk bottles, or my wine bottles not many of those of course I'm a responsible drinker, but I know exactly what to do with them. So what can your average person do with an old phone like that, to make sure that they are safeguarding our element future?
Lizzy - So there are some things that you can do the Recycle Now website has a facility but you can pretend your postcode and it will tell you what recycling facilities nearby.
Chris - But are there any?
Lizzy - What we found was there's there's not. I've tried, and there's not consistency across different councils.
Chris - Because the Royal Society of Chemistry are a pretty potent organisation, they can lobby very hard. Is this something that you're going to start pushing this year and say look we need to be more responsible about this we might have, you know it sounds like a long way away 50 years, but time is passing quickly and we're going to run out of these things pretty fast.
Lizzy - We're doing a piece of research right now to demonstrate the scale of the problem and find out how many people are hoarding devices. And we'll be able to start producing some advice for government, manufacturers, retailers and individuals on how we can protect these basic elements better in future.

47:10 - Elements of the future
Elements of the future
with Kit Chapman, Chemistry World magazine
What might elements, and the periodic table, look like in the future? Katie Haylor spoke with Kit Chapman, author and comment editor for Chemistry World magazine, and author of Superheavy: making and breaking the periodic table. First up, Katie asked, is there actually space for more elements in the table?
Kit - There's lots of space, don't worry about that at all. At the moment two groups are even working to try and get the next two elements and they're actually in a race at the moment in Russia and Japan. So we're expecting that there's gonna be another two elements hopefully within the next five years.
Katie - Now, are these man-made elements?
Kit - Almost certainly. So they don't necessarily have to be. There are theories saying that you could find those elements on Earth, some of them. But at the moment all the elements we're discovering past uranium, which is element 92, have been first discovered by a man-made project. And so these elements are probably going to be found in particle accelerators and we are going to be making them a single atom at a time.
Katie - So if you're hinting that there may be some naturally occurring ones we just haven't found yet, where might they be? Haven't we looked in lots of places?
Kit - That's a very good question. And we have looked everywhere! In the 1970s we looked in the BART system, that's a tube system in San Francisco, we looked in salt mines, we even looked in churches because there was a theory that you could find it in the lead lining of stained glass windows and you could find traces of those super-heavy elements as we call them. But so far we've not found anything. There was a group from Israel that claimed they found element 126. Again that's never been proven. But in theory there could be some of those elements and this is because of something called magic numbers. So we talk about electrons having shells for atoms. There's a good theory put forward by someone call Maria Goeppert Mayer that neutrons and protons also have these magic numbers of shells and that balances everything out and makes them much more stable. Now these elements that we're finding at the moment, the ones we're making, they last very, very small amounts of time. So element 118 for example lasts less than a thousandth of a second, it’s seven ten thousandths of a second.
Katie - Wow, okay. I'm just trying wrap my head around that! So talking about making new elements, can we just cycle back to how you actually do that?
Kit - Yeah sure. So there are two ways you can do it. The first ones past uranium were made through essentially what we now use as nuclear reactors. So it's something called neutron capture and the idea is that you bombard something with neutrons and through radiation, something called beta radiation, neutron turns into a proton and you move one place up the periodic table. These days we've got a particle accelerator. So you get a giant magnet and you make ions - that's a nucleus of an element like calcium for example, that's element 20 - and you make it go faster and faster and faster and you fire it as fast as you can at a target. And if you collide with a nucleus usually it explodes in something called fission. That's not what we want. What we want is fusion, where the nucleus sort of gloops together and forms a larger atom.
Katie - Is there an upper limit to how many we can make?
Kit - Well we don't know. We think the upper limit is about element 172 or 173, before everything will just become too unstable. So that means we haven't found around a third of the elements that could exist.
Katie - Wow. So there's there's a lot to keep chemists busy!
Kit - There's a lot to keep chemists busy and physicists because it gets really weird as well. Because we're making these things bigger and bigger and bigger, we start getting something called relativistic effects. And that's like Einstein in the theory of relativity. It starts affecting how the electrons behave and things get very, very weird. We could have nuclei is that twist into the shape of a doughnut so there's a hole in the middle. We could see electrons orbiting through the nucleus. And so all of this means that the periodic table gets very, very weird indeed.
Katie - So how weird do you reckon it's going to get? How far are we going to stray?
Kit - It's possible that there might be an area where chemistry just simply does not work. The elements become so strange that they would exist as cations only, as we call it, so it wouldn't be able to attract electrons. Very, very strange! At the moment, with element 118 we're already seeing that weirdness. So earlier we were speaking about how elements in groups have different properties as they go down. Element 118, oganesson, is in theory a noble gas. It should be a gas at room temperature, it shouldn't react very much with elements at all. Oganesson however, we think is very reactive and is actually a solid at room temperature, so it just stops following the rules completely.
Katie - So potentially new discoveries may well break the periodic table.
Kit - We might already have broken the periodic table!

51:49 - QotW - Why are some people so good at accents?
QotW - Why are some people so good at accents?
Adam Murphy set out to find an answer to Lia's question...
Adam - Do you call the letter after Q, “oar”, or “are”? Do you say “Bath”, or “bath”? There are nearly as many accents as there are people who speak. But why does that happen? Jonathan Goodman, from the Cambridge Language Science Department filled us in.
Jonathan - I think there are two layers of answers to this question. The obvious explanation has to do with upbringing, although there’s likely an element of natural talent involved, just like with stage acting. We know, for example, that if you grow up bilingual, you’ll be more effective at speaking in the appropriate accent in either language, and probably in foreign languages generally. So there’s probably an element of exposure to multiple languages (and by consequence, accents) within a particular window — usually about the first ten or so years of life.
Adam - So if you grow up around loads of accents, you might be able to imitate them easier. Maybe all our exposure to American television on this side of the Atlantic might give us an edge. But there’s more to it than that.
Jonathan - The second layer, I think, has to do with the evolutionary origins of human communication. Some research in linguistics and evolutionary anthropology suggests that the diversity of languages can in part be explained by kin (or, according to some, group) selection, whereby the pronunciation of particular words signals a connection with a certain kin or group.
Adam - Like a sports jersey for our vocal chords then.
Jonathan - The classic example is the Biblical case of ‘Shibboleth,’ in which, after a battle between two tribes, the victors distinguished between their own tribe and the enemy by asking everyone crossing a river to pronounce the word, ‘Shibboleth.’ Members of the defeated tribe were known to pronounce the word differently, allowing easy distinction between those in the group, and those not.
Adam - That might be an extreme example, but it shows the importance of accents for being part of a group.
Jonathan - If accents evolved, at least in part, to allow people to distinguish kin from everyone else, we’d expect this to have profound implications for how language evolved and continues to evolve. We might then expect people with more diverse linguistic, or even genetic, backgrounds to be able to emulate foreign accents more effectively — but until more extensive research is done, we can’t say for sure.
Adam - Thank you Jonathan for lending your voice to that. Next week we’re hitting the bar, for an answer to this question from Donald.
Donald - How does ethanol interact with the brain and why does it disproportionately affect the area involved in behaviour and movement rather than the parts of the brain involved in vision, hearing, touch or the brainstem involved in breathing, blood pressure and heart rate?










Comments
Add a comment