Where did the 2009 H1N1 swine influenza pandemic come from? This week we hear the evidence that this new 'flu may have escaped from a laboratory. We also explore rising rates of resistance to the antiviral drug Tamiflu, hear how 'flu vaccines are made and a mutant 'flu strain developed by scientists to protect the population. Plus, why soy cuts cancer recurrence rates, how a case of mistaken identity spells trouble for endangered fish, a computer model for unclogging coronary arteries and in Kitchen Science Ben and Dave measure the speed of a sneeze...
In this episode

Soy stops breast cancer recurrence
Women who eat soy products are much less likely to die from or develop a recurrence of breast cancer.
A paper in JAMA by Xiao Shu and colleagues followed up over 5000 Chinese breast cancer survivors aged 20 to 75 for up to 7 years. The study also involved collecting lifestyle data and dietary information including soy-based food intake.
 After a median follow-up of 3.9 years there had been 444 deaths and 534 disease recurrences. But when the team organised the data according to soy intake the found a striking difference: the women who ate the largest amount of soy-based food per day were nearly 30% less likely to have died and 32% less likely to have developed cancer recurrence compared with women who ate the least. The effect showed a linear "dose-response" relationship, with the protective effect peaking at an intake of about 11g per day. The reason for the protective effect is not entirely clear but scientists suspect that it may be related to the presence of oestrogen-like isoflavone molecules that are present in soy and known as phyto-oestrogens.
After a median follow-up of 3.9 years there had been 444 deaths and 534 disease recurrences. But when the team organised the data according to soy intake the found a striking difference: the women who ate the largest amount of soy-based food per day were nearly 30% less likely to have died and 32% less likely to have developed cancer recurrence compared with women who ate the least. The effect showed a linear "dose-response" relationship, with the protective effect peaking at an intake of about 11g per day. The reason for the protective effect is not entirely clear but scientists suspect that it may be related to the presence of oestrogen-like isoflavone molecules that are present in soy and known as phyto-oestrogens.
Paradoxically, some scientists had expressed concerns that these molecules might actually aggravate breast cancer therapy, particularly for hormone-sensitive tumours which can be oestrogen-driven, but this study shows that's not true. Instead the scientists found that tamoxifen (which blocks the growth-promoting effects of oestrogens on cancer cells) and soy food could improve survival.
"We found that soy food is safe and was associated with lower mortality and recurrence among breast cancer patients," say the researchers. "This study suggests that moderate soy food intake is safe and potentially beneficial for women with breast cancer."

A fishy case of mistaken identity
An endangered fish in the North Atlantic could be in even more trouble than previously thought because it turns out that for a long time it has been mixed up with another, less-threatened species.
White marlin are magnificent, fast-swimming, ocean-going fish that can weigh in at over 80kg and grow up to 3m long, and each one has an amazing long spear on their nose. But they are not doing well. They are the prized target of a multimillion dollar sport fishery in America and caught accidentally by longline fisheries aimed at tuna and swordfish.
 A few years ago it was estimated that the number of White Marlin left in the wild has been driven down to only around 12% of the number needed to maintain a healthy population. But it now seems they could be doing even worse still, because scientists have been confusing them with another species that looks like it, called the Roundscale Spearfish.
A few years ago it was estimated that the number of White Marlin left in the wild has been driven down to only around 12% of the number needed to maintain a healthy population. But it now seems they could be doing even worse still, because scientists have been confusing them with another species that looks like it, called the Roundscale Spearfish.
That's the finding published in the journal Endangered Species Research by an international team of marine biologists co-led by Laurence Beerkircher from the NOAA Fisheries Service and Mahmood Shivji from the Guy Harvey Research Institute both in the US.
By sifting through DNA samples from the past decade they found that almost 30% of fish reported in fisheries statistics as White Marlin, were in fact Roundscale Spearfish, an enigmatic look-a-like fish that we know very little about and only made itself known to science in 2006 when a genetic study identified it as a separate species.
The team also used computer simulations to work out how the abundance Roundscale Spearfish will alter assessments of White Marlin populations in the past and future. Unfortunately, the crucial piece of missing information is whether spearfish have always made up 30% of the catches, or if that proportion has changed over time. Without that information, it leaves scientists with a very hazy picture of how both these fish are doing making it extremely difficult to manage and conserve them properly.
Twice now the White Marlin has been proposed for protection under the US Endangered Species Act (which would put an end to the lucrative trophy fishery), and twice it has been turned down based largely on population studies suggesting they have been increasing in abundance. But now that the Roundscale Spearfish has come onto the scene, we can't be at all sure if those increases really happened.
We may sometimes think of taxonomy - the science of species identification - as rather an old-fashioned pursuit confined to the dusty corridors of museums. Far from it, this study highlights just how important it is for us to understand what species there are in the world, especially the many ocean-dwelling species that we are busy hunting towards extinction.
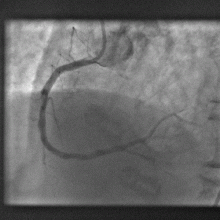
Computer model gets to heart of blocked artery problem
A new computer model promises to dramatically improve the outcomes when clogged heart arteries are unblocked and propped open with metal stents.
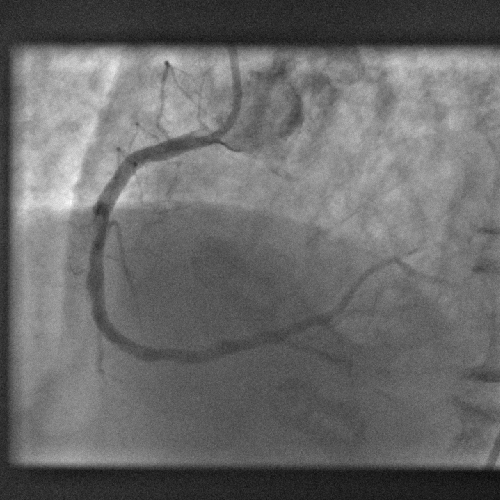 The process of treating heart disease like this, with angioplasty and stenting, known as PCI - percutaenous coronary intervention, has revolutionised the treatment of heart disease; patients now only rarely have to undergo risky open-heart arterial bypass procedures.
The process of treating heart disease like this, with angioplasty and stenting, known as PCI - percutaenous coronary intervention, has revolutionised the treatment of heart disease; patients now only rarely have to undergo risky open-heart arterial bypass procedures.
Instead, tubes are threaded into the coronary arteries via an artery in a patient's leg and dye is used to identify blockages. The narrowed portions of the artery are then re-opened by briefly inflating a balloon inside the vessel before deploying a metal stent, which resembles a miniature cage, to ensure that the artery remains patent.
But when doctors first began to do this they discovered that new cells would grow around the stent, subsequently re-occluding it. The problem was tackled by using stents impregnated with a drug (a so-called DES - drug eluting stent) to block this cell growth. But unfortunately, whilst highly effective, in some cases these stents provoked blood clots and heart attacks in some patients.
Now a team in Harvard have worked out why and how to prevent the problem.
Writing in PLoS one, Vijaya Kolachalama and his colleagues have designed a computer algorithm that can predict how blood behaves downstream of stents and explains why, in some cases, patients have suffered repeat problems. The model shows that when stents are plumbed into sections of arteries close to points where the vessels branch, the change in blood flow causes the drug to build up at certain points along the vessel wall, triggering damage.
According to study co-author Elazer Edelman, "by observing the arterial distribution patterns for various settings, we understood that drug released from the stent does not reach uniformly to all regions of the vessel and this non-uniformity depends on where the stent is placed in the artery as well as blood flow that is entering the vessel." This should make it much easier for doctors to position stents with greater safety in future and may help designers to develop novel stents capable of avoiding these problems. It may also herald the future arrival of individualised stents, bespoke built to match the specific demands of a patient's vasculature.

09:25 - Read my words
Read my words
New computer software can read a book's literary fingerprint that is unique to the author who wrote it...
It turns out that as books get longer, even the very best writers eventually start to run out of new words to use. But, the rate at which new words drop off depends on the skill of the author and is always the same for each author, giving them a unique word-frequency fingerprint.
Based on analysis of books and stories by three great writers, Herman Melville, Thomas Hardy and DH Lawrence, a team of researchers from Sweden, led by Sebastian Bernhardsson, have come up with the idea that inside every writer's head is a 'meta-book'. Writers reach into their own personal meta-book and pull out words to put down on paper or computer screen.
The study published in the New Journal of Physics provides insights into the way language is used especially by the greatest writers.
The findings go against a 75 year old theory, called Zipf's law, which suggested that for any written work, the frequency of any word is inversely proportion to its rank. So, if the word "the" is most frequently used and accounts for 7% of all the words used, the next most frequent word "and" accounts for around 3.5% and so on.
But, rather than a universal literary rule, this new study shows that linguistic ability of individual authors is more important in determining their new-word frequency.
The team is planning to conduct similar research on other literary works, and eventually think it should be possible to pick out an unidentified author from the fingerprint left in their words.
15:03 - Did Swine 'Flu Escape from a Laboratory?
Did Swine 'Flu Escape from a Laboratory?
with Adrian Gibbs, Canberra
Chris - If you look back over history, flu pandemics come roughly over 30 years or so. This occurs when a new strain of the virus begins to circulate in the human population. But where do these new 'flus actually come from? Well, in the past, we've implicated birds. We think they might be partly to blame. But this time, with the 2009 swine flu pandemic, we're not quite so sure. And some scientists think that this pandemic may have even been man-made.
Adrian Gibbs is a virologist, and he's based in Canberra in Australia.
Adrian -  Well, the problem I'm interested in and with my two colleagues is where has this 'flu, this new pandemic 'flu, come from because if you really want to stop a pandemic in occurring, the best thing is to try and nip it in the bud at the very beginning. We've got to try and learn where these pandemics are coming from, what their conditions are. Then we can decide how we can best control them.
Well, the problem I'm interested in and with my two colleagues is where has this 'flu, this new pandemic 'flu, come from because if you really want to stop a pandemic in occurring, the best thing is to try and nip it in the bud at the very beginning. We've got to try and learn where these pandemics are coming from, what their conditions are. Then we can decide how we can best control them.
Chris - What are the present theories about how they arise?
Adrian - Well, the simplest theory that has been put out is that perhaps it's an entirely natural occurrence. We believe that most 'flus are getting around in birds so presumably it's got around in birds. However, this new flu shows no relationship with birds. All of its most immediate ancestors are viruses of pigs. Therefore, you've got to believe where it's got around in pigs. But the real problem with that is that it clearly has three separate parents. And one of those parents was in North America some years ago. One was in South East Asia. And one was in Europe. So you got to think of getting three pigs from different parts of the world together to make the new virus.
Chris - So what you're saying is when you, sort of, molecularly interrogate the genetic history of what we're seeing as the H1N1 swine flu pandemic, you can see that it must have three independent bits of parentage. And the big question is, "Well, how on Earth did all those parents come together to infect the first person who got this?"
Adrian - That's right. So if they were brought together by pigs, for example, say through live pig trade between continents, it means that quarantine, which has worked for dozens of years in the past, has somehow broken down on at least two occasions. If you got to get three things together in one part of the world, one might be there originally. The other two have to come through quarantine to leave the country and come through quarantine to get into a country. So that's one possibility. The other possibility which we have put into this paper that we have published is that we've been thinking, could have they got together by some sort of laboratory error? We know that virologists pass viruses from one another around the world for use in their laboratories, for identification, for making vaccines and so on. What sort of conditions might three viruses have got together in one place in order to be able to shuffle their genes and produce the new pandemic flu?
Chris - That's a fairly scary suggestion though that this thing that has cost the Earth. I mean, quite literally cost the Earth in terms of the number of people who have caught it, days off work, pandemic preparedness, giving people antivirals, preparing a vaccine. The cost is tremendous and if that is man-made, that's a terrible thought.
Adrian - Yes, that's certainly true because the data as it stands at the moment doesn't distinguish between a man-made problem in the laboratory and a man-made problem in the quarantine. And the reason is that the gene sequences that are being analysed to produce these results are all from more than 10 years ago. Ten to 17 years ago was the last time that those particular sequences were seen. So there's this big gap in the middle and what is actually required then is to try and try those intermediate sequences and then we might be able to distinguish between these two theories.
Chris - I see. So what you're saying is that when you look at the present-day pandemic genetic sequence and you ask, "When did the parent sequences that must have made that pandemic last circulate somewhere in the world?" The last time they were reported or picked up anywhere was more than 10 years ago.
Adrian - That's right. That's a long gap. So we're not sure. We know that those parents were in these three places. North America provided six of the genes of this flu and one gene each came from Europe, and another one came from South East Asia. We should obviously then institute a search for these and probably some people are looking for them. For example, there are many parts of the world where pig populations are not being sampled for swine flu, for example, South America. The other possibility is that it's in one of the labs that has these three strains of virus in it. And one, at least of those strains is very close, if not identical, to a strain which is commonly used in swine influenza vaccines. That's vaccines for pigs to protect them against flu. And so, there's a real possibility that it came through vaccine manufacture and probably not even known to the people who have done it.
Chris - So how would something like the new H1N1, if it were the progeny of a vaccine being made, how would that get out of the lab and then into the human population? What would be the mechanism?
Adrian - Well the simplest of all the possibilities is that in the making of one of these swine vaccines which are often multivalent that is that they've got more than one virus in the vaccine. So what they do is they grow up each virus individually, take the particles in the virus which were going to make the vaccine, put them together, and then kill the virus by adding formaldehyde or propionolactone. This sterilises the virus. They put this mixture then at three dead viruses into the pig. The pig then becomes immune. But if for example, one unfortunate morning somebody forgot to put the sterilant into the mixture, then a pig will be simultaneously infected with three viruses. And those three viruses might grow, shuffle genes and have produced the new virus. So that's the simplest possible mechanism. But there are other possibilities.
Chris - Which are?
Adrian - For example, we know that flu can grow in cultured cells. Many laboratories are now going over to using cultured cells to do the flu work. And there's a paper published saying, that if you put flu into these cells, it grows with it. And you can detect it. But eventually it becomes latent in those cells. Now those cells are passed from lab to lab. And so it could be that a cultured cell, which is being shared between laboratories, is carrying the virus between laboratories. And so again, it's entirely feasible to think of scenarios in which the three viruses could've got together and end up in the laboratory.
Chris - It's feasible. Yes, sir. Is it realistic, though?
Adrian - It is realistic. There are historic examples. We know the viruses get out of laboratories. I mean, you have had this terrible epidemic of foot and mouth disease in Britain recently which cost the country millions and millions of pounds. But there are examples even from flu. There was a flu virus circulating in the human population which disappeared in 1957. And then it reappeared in 1997. And the one that appeared in 1997 had not changed at all from when it disappeared in the 1950s. And so it had not being growing. It had been hidden away, frozen somewhere perhaps. And so the safest conclusion is that it was in a laboratory somewhere. You had a young person working in the lab, who had not got antibodies against the earlier strain, who got infected and then spread it out into the community. So there are precedents.
Chris - Quite scary though, those precedents, Adrian. Do you think, therefore, that what we should do is to institute better regulation to make sure this doesn't happen again?
Adrian - I'm sure that that would be a very good idea. And another thing which would help restore confidence in what's going on is that laboratories should be required to keep a register of all the viruses that they have in stock. So when for example, a new pandemic occurs, the WHO could have already in its possession a list of all of the viruses that are being studied.
Chris - But meanwhile, scientists are still looking for the source of this year's swine flu. And who knows, it could have come from a laboratory. It's quite scary to think it could've been man-made. That was Canberra-based Virologist, Adrian Gibbs.
23:37 - What Causes Tamiflu Resistance?
What Causes Tamiflu Resistance?
with James Wood, Cambridge Infectious Diseases Consortium
Helen - Many countries have responded to the swine flu pandemic this year by giving people with 'flu symptoms a course of an antiviral drug called Tamiflu - oseltamivir. But, now we're seeing patients with 'flu infections that are resistant to this drug. To explain why this happens and what the consequences might be, is Professor James Wood, director of the Cambridge Infectious Diseases Consortium. I thought perhaps we should start off by just going into some detail about how Tamiflu actually works.
James - Yes.  This is one of the two sets of proteins of influenza and it stops or dramatically inhibits the production of infectious viruses from infected cells.
This is one of the two sets of proteins of influenza and it stops or dramatically inhibits the production of infectious viruses from infected cells.
Helen - Right. So how is it that then we see some resistance occurring.
James - The use of any [anti-microbial] drug, just as with antibiotics in patients with a bacterial infection, creates a lot of pressure on the infection to evade the drug that's being used. And although viruses aren't exactly living, they behave like living beings within cells because they have replication machinery that allows them to change very quickly; so they make very simple genetic changes, which can occur by chance, and this creates a new strain of the virus that's not sensitive to the drug.
Helen - So the nuts and bolts of this is the genetic mechanism by which viruses work. We get mutations, and that's when the resistance comes in.
James - That's absolutely right. And that's very similar to the development to any drug resistant to either viruses or bacteria.
Helen - So it was just a matter of time until we saw some resistance coming up to Tamiflu?
James - I think so. And I think what matters is whether or not the drug resistant form of the infection is transmitting widely.
Helen -  Do we have any idea if it is?
Do we have any idea if it is?
James - Well, I think at the moment that there are a few special cases where drug resistance has been detected, but it's not being transmitted widely in the community and most strains that people have been infected with are still not resistant.
Helen - Do we understand what it is about a particular resistance pattern that makes it become widespread or not or is that another big question mark?
James - Well, I think that's a big question mark; many changes that are occur in many infections are frequently deleterious [to the fitness of the virus]. So the change towards Tamiflu resistance actually can make the virus slightly less good at transmitting. Therefore you're more likely to catch a form of the infection that's not carrying the Tamiflu resistance. But you might expect to see widespread transmission of resistance when the drug is being used very, very widely.
Helen - And so, are we seeing it as a big problem in the U.K. at the moment?
James - What we're finding is that there's not a significant problem at the moment; most of the people that are treated with Tamiflu at the moment are not infected with resistant virus.
Helen - So we see this resistance occurring and perhaps to some extent it's a little bit inevitable that it will happen. What can we do about it?
James - Well, I think that one of the most obvious ways is to try and make sure that people who are receiving Tamiflu, but are still infected, are not walking around in very high risk situations in terms of transmission. So a degree of quarantine would be a very effective means of stopping people transmitting drug resistant virus.
Helen - So ultimately, give someone Tamiflu, keep them on their own until we know that they've got better and there's no problem.
James - I think that's absolutely right. I mean, we're not talking about in long lived infections of influenza. The majority of people have stopped shedding large amounts of virus within a few days.
Helen - So thanks, James. That's Professor James Wood, Director of the Cambridge Infectious Diseases Consortium.
27:51 - How are 'Flu Vaccines Produced?
How are 'Flu Vaccines Produced?
with Tarit Mukhopadhyay, University College London; John Dillon, GlaxoSmithKline; Rino Rappuoli, Novartis
Chris - This week, Meera Senthilingam has been down to find out how flu vaccines work and just what's involved in making them.
Meera -  In light of the current swine flu pandemic, pharmaceutical companies have had to step up the production of their vaccines in order to protect the population. But just what's inside a flu vaccine to give us immunity to this potentially deadly virus and how are they kept up to date with what's currently circulating in our population? To find out, I met Tarit Mukhopadhyay from University College London.
In light of the current swine flu pandemic, pharmaceutical companies have had to step up the production of their vaccines in order to protect the population. But just what's inside a flu vaccine to give us immunity to this potentially deadly virus and how are they kept up to date with what's currently circulating in our population? To find out, I met Tarit Mukhopadhyay from University College London.
Tarit - So the main component of every flu vaccine is the HA - haemagglutinin - protein. It's a surface protein on the top of the virus. It's split from the virus then purified and made safe for injection into human beings. It's this protein that forms the basis of the immune response. So the immune system recognise this protein, assimilates it, and then should you be infected by the influenza strain carrying the same protein, it will seek to attack it and destroy it.
Meera - What are the main challenges facing a flu vaccine production?
Tarit - So I guess the first challenge is producing the vaccine strain, the production strain of the influenza. There are two techniques that you can use. One is classical re-assortment and the other is reverse genetics. With the re-assortment, what you want to create is a high-yielding production strain that encodes for the HA protein that's circulating on the the wild-type strain. With reverse genetics, because of our ability to manipulate genes the way we can, we essentially engineer six plasmids for the production strain and two plasmids that will have the HA and NA - neuraminidase [another viral surface protein] genes; these are combined in a cell to produce the virus of interest.
Meera - So essentially, the production strain that's generally used is something that's good at reproducing. And then this is being combined with a strain that's in circulation and found in our environment at the moment so that you get a high-yielding vaccine that will stimulate the immune response that you want.
Tarit - That's exactly right. So you want those virus components, those virus surface proteins that will help to stimulate the immune system. But you also need to have them in a format that can grow well in whatever culture method you are using.
Meera - Tarit Mukhopadhyay from University College London. And as Tarit mentioned, once you have the strain of virus that you want to replicate, it then needs to be cultured to multiply the amount you have available for vaccines. Chicken eggs have been used for tens of years. They're a trusted and known method used by many pharmaceutical companies including GlaxoSmithKline (GSK). Explaining more about this egg-based technique, here's John Dillon, medical director of GlaxoSmithKline's Pandemic Center of Excellence.
John - Well, we use millions of eggs; we take the seed [production viral] strain and we inject it into the eggs, and the eggs will replicate the virus so that you get lots of copies of the virus within the eggs. And then we essentially harvest them, purify it and we then inactivate the viruses, and we make sure that we've got enough antigen as a result of the inoculation of all of those millions of fertilised eggs.
Meera - When the chick eggs are ready to be injected with these viruses? So what age are they used?
John - So the fertilised embryos are usually around 10 or 11 days after they are injected. Then the viruses are allowed to grow for several more days before the virus is then harvested from the eggs.
Meera - It's obviously quite labour intensive. It requires a lot of chick embryos. So how do you, kind of, scale up this process when a pandemic occurs?
John - I can't pretend that it has been a major effort and ramping up to that level of production - probably a tenfold increase - is part of the challenge overall of trying to respond to a pandemic. But we have been prepared for it. You know, we're now in a situation where we're able to fortunately meet the demand that has been created by governments around the world.
Meera - John Dillon from GlaxoSmithKline. So whilst the use of chicken eggs has helped us to meet the demand for both our seasonal and pandemic flu vaccines, it still has its limitations. And as a result, companies such as Baxter Pharmaceuticals and Novartis have begun using cell culture methods as well. And so to find out more about this newer technique, I spoke to Rino Rappuoli, head of vaccine research at Novartis.
Rino - The technology that we came up with is based on culturing the virus in mammalian cells, growing in fermentors instead of using eggs. We let the virus grow into the cells and then, about 48 hours later, when the virus multiplied to very high yields, we harvest the virus, purify and then kill it. Then we purify the essential components of the virus which are important to make a vaccine, which are the haemagglutinin (HA) and the neuraminidase (NA). In a week we can be ready for large scale manufacturing.
But the majority of H1N1 pandemic influenza vaccine production is still largely being carried out using egg-based influenza vaccine. For the future, I see cell culture will slowly become the method of choice. But today, for this pandemic, we are still largely dependent on egg-based manufacturing.
Helen - That was Rino Rappuoli from Novartis Pharmaceuticals and before that, University College London's Tarit Mukhopadhyay and GSK's Medical Director, John Dillon speaking to Meera Senthilingam about the challenges facing flu vaccine production today and how this looks set to change in the future.
Should you "feed a cold and starve a fever"?
Chris - Well, actually, it's part right, part wrong. Mostly part wrong. The "feed a cold" is absolutely right. A "starve a fever" is probably absolutely wrong. There was a paper that got published and we reported it on The Naked Scientists last year - in the Physiological and Biochemical Zoology journal by Lynn Martin. She reported taking deer mice and starving them, in other words, they gave them 30% fewer calories than they would normally need in a day. The mice didn't show any obvious behavioural differences compared with well-fed mice. But when they did blood tests on them they had far fewer memory B cells. These are the cells that make antibodies that defend you against infections in the future. So this shows that if you aren't getting a good enough diet you will therefore have too little energy to put into mounting an effective immune response and therefore you'll be more prone to an infection in the short term and more prone to an infection subsequently as well. This also fits with other studies that have been done where people receiving measles vaccine have been followed up. People who weren't eating enough had far fewer anti-measles antibodies subsequently compared with people who are much better fed. So, you should always 'feed a cold' is the moral of that story, but you should definitely not "starve a fever."Helen - I just try and eat a thing, basically. And if you're feeling awful, try and get something down. Exactly.Chris - Basically, you need energy to master your immune response. You have to grow lots of cells. And that takes energy. And you got to feed yourself.Helen - Makes sense.
35:20 - Protecting, Anti-'flu Viruses
Protecting, Anti-'flu Viruses
with Nigel Dimmock, Warwick University
Chris - We've heard about vaccines offering one way to combat flu. We've also got antiviral drugs like Tamiflu to fall back on. But, as we've heard, the virus can mutate. And some of those forms can become drug resistant. So is there anything else we can do? 
Well, Dr. Nigel Dimmock is a researcher at Warwick University and he's looking at a different antiviral approach. He's infecting people with what are called protecting viruses. And he's with us now. Nigel, what is a protecting virus?
Nigel - Well, Chris, a protecting virus is something which is absolutely new in terms of treating virus infections. The lovely thing about protecting viruses is that they are actually made by the virus itself. It's a big mutant of the virus. Now, the thing is that all viruses make them as I say. And they are antiviral.
So what we get is a virus, a perfectly normal-looking virus, but it has a genome from which a large chunk has been excised by some accident of replication. Now, what happens is that when this gets into a cell alongside an infectious virus, the infectious virus multiplies itself. But it also multiplies the defective form which we call the protecting virus. And because of the dynamics, there's much more of the protecting virus made than of the infectious stuff. So what happens is that the protecting virus swamps out the infectious virus. The virus is aborted and you get better. And it essentially buys time for the immune system to mop up the infection.
Chris - So, in a nutshell, you have a flu virus replicating. Normally, it's got to copy all of its genetic material making new progeny viruses. These come out and infect other cells and when they've infected lots of cells, that makes you feel sick and you've also got the opportunity to infect other people. With the protecting virus, it's a modified form of 'flu which can arise by chance but which has defective genetic material and is easier for it to grow than the follow on normal flu. Therefore it gets in the way, puts a spanner in the works, so you make less flu. You are less infectious and you are less infected and therefore, you don't feel as bad.
Nigel - That's absolutely right. And there are various very nice things about this; one is that you could be treated with this protecting virus by, for instance, a nasal spray. Now remember, it is flu virus. So it knows which cells to go to. It goes to exactly the same cells as the regular flu goes to but it's not infectious. So, if that happens, then the protecting virus genome will sit in this cell. And it will slowly decay, but a single dose will provide you with protection for certainly a number of days and perhaps weeks.
Chris - Presumably also, Nigel, because of the way flu works with all of its genes as little miniature chromosomes, these so-called ribonucleic proteins, it doesn't actually matter what strain of flu you'll get infected with. This interfering or protecting virus will basically protect you against virtually any type of flu.
Nigel - Yes. This is one of the other very good points about protecting virus, is that unlike the vaccine which is be absolutely specific for the strain against which it was made, this protecting virus will protect you against any strain of flu A. You don't even need to know what you're infected with as long as it's flu A; the protecting virus will protect you willy-nilly.
Chris - And presumably, the other benefits is that you will make some antibody against a flu anyway which will help. But doesn't that mean then that if someone already has antibodies - because they've already had a dose of the flu in the past - they might not get very well infected with your protecting virus? And therefore they wouldn't get any benefit from it because they're already immune.
Nigel - Yes, this is a technical point which means that you have to choose a virus to deliver the protecting genome which people haven't had. So what we're using is actually a strain which was isolated in 1934, one of the very first flu strains to be isolated. And it's the same one that is used as the work horse as we've heard earlier in the production of vaccines. So there are not many people who were around in 1934 around now. And if they were, their antibodies are rather decayed. So it's a perfect delivery vehicle.
Chris - That's all very well. But then once you've used it once, those people presumably can't derive benefit again because they will then be immune. So you'll have to change the virus, won't you?
Nigel - No, that's not right. No. We give a very low dose. It's a far lower dose than you'll get with the vaccine. And this is another key point about protecting virus because it does work at a very low level. So you don't need to produce as much as you would with the vaccine. And because it's at this low level, it's not stimulating immunity.
Now, when you get infected, the virus that is produced is the infecting virus. Not the virus that delivered the protecting genome. So you're not producing more of the protecting virus but you're producing simply more of the incoming infectious virus. So the immunity will be against the infectious virus, not the protecting virus.
Chris - Which sounds encouraging. And just to finish off, can you tell us when we might be able to see this actually being used because obviously, this is an experimental tool of the moment. To my knowledge, this has not gone into patients, has it?
Nigel - No, that's absolutely right. We've done a huge amount of lab work. It works very well under experimental situations. But the thing now is to get it into human clinical trials. As you are aware this is a hugely costly business and what we're engaged with now, with the university, is in raising money to carry out these clinical trials. And then we shall really know whether protecting virus is a real prospect for human medicine.
Chris - So if anyone wants to write you a cheque, just write your cheques out to Nigel Dimmock in Warwick University... I'm just joking, Nigel. But thank you very much for joining us. It's been great having you on the show.
Nigel - Absolutely.
How many 'flu-vaccine doses from an egg?
Chris - The answer is that you put the vaccine virus into the egg, which has a chick inside. The chick turns into chicken soup with some 'flu added. You get the 'flu out, purify it and you end up with about three doses per egg. So, if you do some maths, if you want to immunise the entire population of the U.K. with, let's say (for "eggs-ample"), 60 million to make the numbers easy, you would need - just for the U.K. - 20 million laboratory grade chicken's eggs. So a lot is the answer. In terms of egg-safety, the eggs used are laboratory grade eggs. They are high-grade, disease-free, guaranteed to be safe chickens' eggs. And also, the testing that goes on is incredibly rigorous. The eggs are tested, the vaccine progeny is tested, and what comes out is tested to make sure that there's nothing in there that there shoudn't be...
How does 'flu infect cells?
Chris - The way flu gets hold of cells is down to proteins on the surface of the virus particle. Each virus particle is tiny, about 1/10,000th for the millimetre across. If you could zoom in on the surface, you'd see that it had these spikes on the surface. These spikes are a structure called haemagglutinin, which is a tiny protein resembling a molecular grappling hook. It's viral Velcro. It gets hold of something called sialic acid, which is a chemical on the surface of the cells that line our nose and throat, and this enables the virus to grab hold of those cells and pull itself in very close. Through this interaction the cell thinks the virus is something that it has to take inside the cell. So the cell then does something called receptor-mediated endocytosis, which basically means it pulls the virus inside the cell. Once inside the virus releases it's genetic material and productively infects the cell. It's a bit like a Trojan horse actually because the Trojan horse was this juicy tidbit sitting outside the gate of Troy. The guys inside the city thought, "Wow! That looks fantastic. We'll pull that inside because it looks good." And it goes inside the city. And then of course, lurking inside are all these people who are then wreak havoc inside the city. And that's basically what a flu virus does. It hijacks the cell, turns it into a virus factory and then it infects all of the cells around it and all the people around you!
Why is 'flu more prevalent in winter?
Chris - Well, we think flu spreads better in winter because of human behaviour because it does this reproducibly in every country in the world and in which it is winter time - it doesn’t mean it goes away completely in summer but it does come much more commonly in winter.
We think that’s because it spreads better in winter because of what humans do. We go indoors more in winter so there are more people together indoors with the windows closed. Also, unlike summer time, it’s less light and therefore there's less ultraviolet radiation to dry out the virus and kill it. So 'flu finds it easier to persist on surfaces spread by coughs and sneezes, and it hangs around for longer.
As a result you have a higher chance of passing it on so that’s what we think goes on. And then the big determinant, the disproportionate determinant, is the school year. The long summer school holiday powerfully knocks 'flu on the head because kids stop mixing and spreading the infection amongst themselves. What normally happens is that they become infected and then go home and give it to their parents and the parents then carry the infection to all of the other parts of the social and age strata, usually through their workplace.

51:58 - What would happen if you Tasered an elephant?
What would happen if you Tasered an elephant?
We put this to a real elephant catcher in Africa, Don Grobbler, standing on the top of a sand dune in the wilds of Mozambique when he gave this answer:
Elephants do not like any electric shocks and they will usually react quite severely to them. We use electric fencing in a number of wildlife reserves in Africa. The electric fencing running around an enclosure is generally in the region of 9000 to 12,000 volts, whereas a stun gun will put out about 50,000 volts. Elephants are taught about an electric shock by putting it in a small pen that's about two acres in size for about 24 to 48 hours. When they've been in contact with an electric fence in that situation, they will never touch an electric fence again. We also use electric prodders to move elephants into a truck to transport them. A taser certainly wouldn't floor an elephant but it might scare it. And in most cases, the elephant would try to move away from a stun gun but I wouldn't want to be there if the elephant decided to take some revenge.
How do disinfecting hand-rubs work?
Chris - It depends what virus you're dealing with. If you're dealing with 'flu, they work quite well because 'flu is what's called an enveloped virus. On the surface of the virus particle is an oily bag which, if you add alcohol from a hand rub or cleansing gel, breaks it apart, denaturing the virus. If, on the other hand, you're playing around with something like norovirus, the winter-vomiting bug, this does not have one of these oily envelopes around the outside of the virus; these are known as non-enveloped viruses. Instead they comprise a very tough particle assembled from proteins linked together. As a result of it does not respond to alcohol in disinfecting hand rubs. These are impervious to the alcohol in hand rubs, so these sorts of cleansers are not effective against viruses like noro. All all you get is a pure culture of norovirus on your hand after using one! Instead, the way to deal with these is actually soap and water; not because the soap kills the noro but by rubbing the skin actually detaches it and this gets rid of it.











Comments
Add a comment