Murder, terrorism and suicide...These are the scenarios generally associated with cyanide poisoning. One would hope these are rare occurrences but incidences of cyanide poisoning are all too common and increasing. The reason for this is that when man-made polymers such as polyacrylonitrile, nylon and melamine are burned they produce hydrogen cyanide (HCN) gas.
The reason for this is that when man-made polymers such as polyacrylonitrile, nylon and melamine are burned they produce hydrogen cyanide (HCN) gas.
These substances are used in clothes and furnishings and so HCN gas can be produced during a fire and anyone near the fire can is exposed to it. Hydrogen cyanide was first isolated in 1782 by Scheele. He later went on to provide a practical demonstration of its toxic effects by accidentally becoming its victim. It is often associated with the smell of bitter almonds but in fact only about 40% of people can smell it.
Cyanide is naturally present in everyone's blood in very small amounts, and people who smoke tend to have more in their blood than people who don't.
This would be a good moment to define 'cyanide'. Cyanide is the negatively charged ion, CN- but at physiological pH 7.4, when unbound, this is in the form of hydrogen cyanide, HCN. Once cyanide is taken into the blood stream the majority (92-99%) is found bound to hemoglobin (Hb) in red blood cells. From there it is taken to the body's tissues where it binds to an enzyme called cytochrome oxidase and stops cells from being able to use oxygen. The signs and symptoms of cyanide poisoning range from headache, difficulty in breathing and vomiting to unconsciousness and death. People who have recovered from cyanide poisoning do not usually suffer any long-term effects.
Cyanide can be metabolized rapidly and is generally converted to thiocyanate by an enzyme called rhodanese. Thiocyanate is much less toxic than cyanide and the body can then get rid of this. But (there had to be a but!) the enzyme needs another chemical, thiosulfate, to be able to do this and this can be used up quite quickly.
Cyanide and cyanide containing compounds are used in lots of industrial processes such as electroplating, chemical synthesis and fumigation. Some food types contain compounds called cyanogenic glycosides which can be converted to cyanide in the body; these include cassava roots, lima beans and bamboo shoots. In addition drugs such as sodium nitroprusside, sometimes used for the treatment of hypertension and Laetrile, an anti-cancer agent, also release cyanide into the circulation.
Cyanide salts are the forms which historically have been used for suicide and murder. They can't be bought as easily now by the public and so they aren't used much for this now. Nevertheless there are recent cases, for example in 1982 seven people in Chicago were killed by Tylenol tablets (painkillers) which were spiked with cyanide salts.
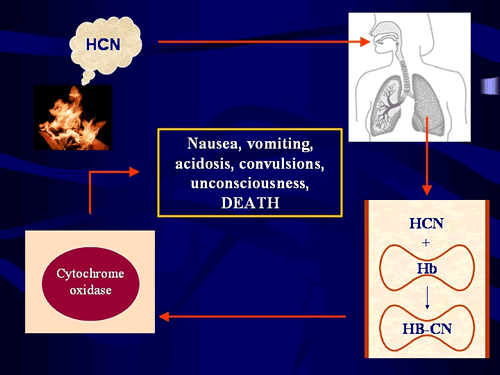 |
| Figure 1: The effects of cyanide within the body. Hydrogen cyanide gas (HCN) is inhaled and locks onto haemoglobin, the oxygen-carrying molecule in red blood cells (bottom right). It is then distributed via the bloodstream to cells throughout the body where it binds to an important metabolic enzyme called cytochrome oxidase (bottom left), preventing cells from using oxygen to produce energy. In this way cyanide effectively chemically asphyxiates the body. |
There are antidotes available for cyanide poisoning but they can have bad side effects or be toxic themselves. For example some drugs act to change normal hemoglobin to another form called methemoglobin, as cyanide has a high affinity for this type of hemoglobin, which binds the cyanide and stops it from reaching the tissues.
Unfortunately oxidized hemoglobin does not bind oxygen and so obviously it is not desirable to have too much of this form present. Extra thiosulfate can also be given so that more cyanide can be metabolized, this is often used with methemoglobin formers. Cobalt (II) compounds form cyanide complexes and these are used but they are highly toxic. Hydroxycobalamin (Vitamin B12a) which forms cyanocobalamin (Vitamin B12) has lower toxicity but it is not widely available. Due to the toxicity of these drugs it is important they are given in doses related to the amount of cyanide in the body.
So, yet another reason to make sure you've checked the batteries work in your smoke alarm!
References
- Previous Creationism vs. Science
- Next Genes for Bigger Brains










Comments
Add a comment