Exploding Eggs - Burning Hydrogen
Ingredients
When hydrogen reacts with oxygen (burns) it releases a huge amount of energy as it forms water (H2O)
2 H2 + O2 -> 2 H2O + lots of energy
This can be seen in a couple of rather gratuitous explosions that
Dr Hal did when he came to visit.
He then took an egg which he had blown the contents out of. It was emptied through a hole at one end by blowing through a second hole at the other end.
The egg was filled with pure hydrogen and the top was lit.
He then repeated the process using a much stronger ostrich egg.
Explanation
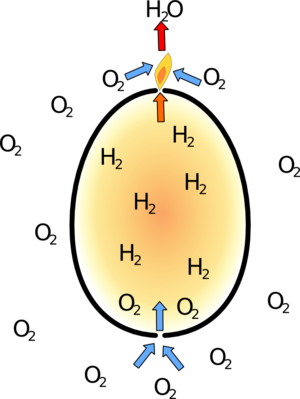
Hydrogen is less dense than air, so it will float upwards through the top hole in the egg. This escaping gas can be lit quite easily. The flame can't burn inside the egg to start with because there is no oxygen there.
 |  |
|---|---|
| If egg filled with hydrogen through two holes, the gas will float out of the top and can be lit. Air will move into the bottom of the egg to take its place. | The hydrogen flame at the top doesn't spread into the egg for the moment as there is not enough oxygen in the egg to support combustion. |
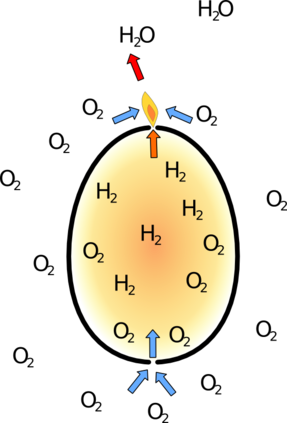
However the hydrogen is only floating because the more dense air is pushing it upwards.
So the egg slowly fills with air until the flame can burn inside the egg. At this point all the remaining hydrogen ignites inside the egg. This releases lots of energy and heats up the gasses in the egg to hundreds of degrees celcius.
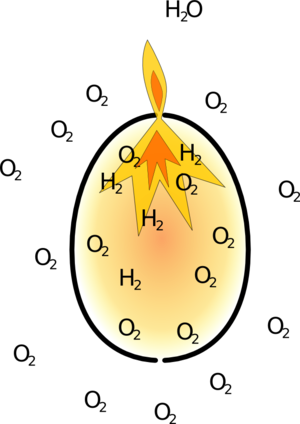
Hot gasses take up more space than cold ones so they push hard on the sides of the egg until it fails explosively. The stronger the egg the higher the pressure it fails at so the more energy is transferred to the splinters. This is why the ostrich egg is so much more violent.
 |  |
|---|---|
| Eventually there is enough oxygen in the egg for it to ignite. | The energy released by the reaction causes the gasses inside the egg to heat up, and expand violently. The egg can't take this force and breaks into pieces. |
- Previous Phosphorus Moon
- Next Light Bulbs in Liquid Nitrogen










Comments
Add a comment