How to make a forcefield
This week Derek and Dave are venturing bravely into the future to make their very own forcefield. Providing the man power to do it are Matthew and Robbie from Campers Playscheme, which is held at Hunsbury Park Primary School...
To do this experiment, you will need:
Empty coke can Piece of polystyrene A full head of hair
How to do the experiment: 1 - Take the polystyrene and rub it on your hair for about one minute. 2 - Wave the polystyrene near the fizzy drink can and see what happens.
So what's going on?
When the polystyrene is placed near to the can, the can moves towards you. Why is this?
All materials contain electrons; tiny particles that are part of an atom. Electrons from one material can be passed to another material when they come into contact. It's a little bit like headlice: if one person has headlice (with the lice representing electrons) and their lice-free friend brushes past, then headlice can be passed from one to the other. If they keep their heads together long enough, then there's a chance that the rogue headlice will crawl back again. However if they quickly pull their heads apart, then the headlice have no way of returning to their original owner. This results in an overall headlice gain for the previously lice-free friend.
The same principle can be applied when thinking about the transfer of electrons: when two materials are brought together, electrons can move from one side to the other (and potentially back again). Whether electrons have enough time to get back again will determine whether a material ends up with more or less electrons than it started with.
Now it's all very well and good that materials swap a few electrons, but some materials will on average take a lot more electrons from other materials than they give back. These electron-loving materials can take an especially large amount of electrons if they only come into contact with the other material for a short amount of time - they take lots of electrons but the connection is broken before they can return to their rightful owner. An example of a material that behaves in this way is polystyrene (although polystyrene likes gaining electrons, whereas using the first example, no - one likes to get headlice!)
The perfect partner for an electron-loving material is one that wants to get rid of electrons. An example of this is hair. Each strand of hair donates some electrons as it comes into direct contact with the polystyrene. As soon as the polystyrene is rubbed on another part of your head, the contact with the original strand of hair is lost and the electrons can't jump back again.
The overall effect is a slow accumulation of electrons on the polystyrene, and this gives the polystyrene a negative charge. The charge is negative because electrons are negatively charged particles.
Once you've stopped rubbing your head, what you're left with is a negatively charged piece of polystyrene full of electrons that have been 'stolen' from your hair. But why does this attract coke cans?
Playing with magnets demonstrates that like-charges repel - ie: negative repels negative and positive repels positive. When the negatively charged polystyrene is placed near the coke can, it forces all the electrons in the metal as far away as possible (to the far side of the can). As the electrons in the metals move away, it makes the side of the can nearest to the polystyrene relatively positive. Positive and negative charges attract, which is why the coke can is pulled towards the polystyrene.
It is important to note that polystyrene is an insulator and coke cans are made of metal. Electrons in insulating materials can't move around very well, which is why they are bad conductors of heat an electricity. In contrast, electrons can move around very easily in metal, making it perfect for electrical wiring and saucepans.
When the coke can and the polystyrene are brought together, the electrons in the polystyrene pretty much stay where they are (because they find it difficult to move) while the electrons in the metal can whizz round to the other side. If the electrons didn't move to the other side, the can wouldn't polarise (have a positive and a negative side). If there is no polarisation, then the can won't be attracted to the polystyrene.
If you put the polystyrene near to another insulator, such as a plastic bottle, the bottle would not be attracted. This is because the electrons in the plastic cannot move easily, the material cannot polarise and thus the bottle stays where it is.
Ingredients
![]() Empty coke can Piece of polystyrene A full head of hair
Empty coke can Piece of polystyrene A full head of hair
Instructions
1 - Take the polystyrene and rub it on your hair for about one minute. 2 - Wave the polystyrene near the fizzy drink can and see what happens.
Result
When the polystyrene is placed near to the can, the can moves towards you, you will have to be careful to pull the polystyrene away fast enough once the can starts moving.
Explanation
All materials contain electrons; tiny particles that are part of an atom, when these electrons move we call it electricity. Electrons are negatively charged and the centre (nucleus) of the atom they are part of is positively charged. Positive and negative charges repel themselves and attract each other very strongly so normally there are the same number of each in an object and it isn't charged.
If you touch two materials together sometimes some electrons will get transferred from one material to the other and get lost in it. This will leave both objects charged one positive and one negative. This depends on the material some will tend to gain and others loose electrons. Hair doesn't really mind, rubber likes loosing electrons, and polystyrene likes gaining them.
Although you don't need to rub the two materials together to have this effect, it often helps to increase the area that has been in contact.
To start off the can is uncharged
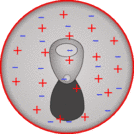
So when you rub the polystyrene on your head it becomes negatively charged. The can is made of a metal (either alumninium or steel), in metals the electrons can move around easily - they are good conductors - so if you put the negatively charged polystyrene next to the can the electrons will be repelled and move to the far side of the can.
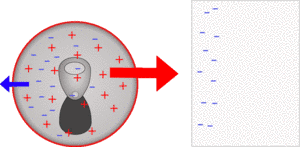
So near to the polystyrene there are lots of positive nuclei which are strongly attracted to the polystyrene and on the far side there are negative electrons which are further away so less strongly repelled, so overall the can is pulled towards the polystyrene.
If you used something insulating like a lemonade bottle instead of the can, the electrons can't move between molecules, so they really can't move very far and the attraction is much weaker.
- Previous White bread and the wonder of enzymes
- Next Strange Temperatures










Comments
Add a comment